Article contents
Modeling of equilibrium conformation of Pt2Ru3 nanoparticles using the density functional theory and Monte Carlo simulations
Published online by Cambridge University Press: 23 February 2017
Abstract
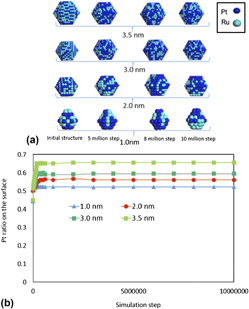
In this study, the density functional theory (DFT) and Monte Carlo (MC) simulations were conducted to determine the equilibrium conformation of Pt2Ru3 nanoparticles with diameters 1.0–3.5 nm at finite temperature. DFT calculations were carried out to estimate the binding energy using slab configurations and energy could be correlated with some structural descriptors and multilinear regression equations to calculate the binding energy from descriptors related to the number of a specific bond to neighboring atoms. MC simulations were carried out to obtain the equilibrium conformation of atoms in Pt2Ru3 at 150–363 K. MC simulations’ result shows that atoms of the same element tend to segregate each other, and Pt/Ru ratio on the surface increases with increasing particle size; also, most of the Pt are located on the surface whereas most of the Ru are located on the subsurface or at the core sites. It is qualitatively exhibited that the Pt/Ru ratio on the surface decreases with increasing temperature.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Susan B. Sinnott
References
REFERENCES
- 7
- Cited by





