Article contents
MnFeTiOx/attapulgite catalysts with excellent potassium resistance for SCR of NOx with NH3 at low temperatures
Published online by Cambridge University Press: 26 February 2019
Abstract
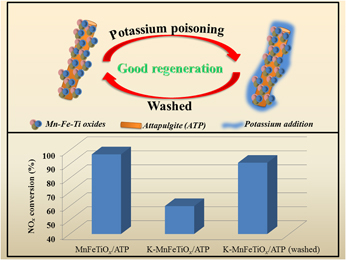
A series of metal oxides (MnFeOx, MnCrOx, MnTiOx, and MnFeTiOx) supported on attapulgite (ATP) were synthesized by coprecipitation for the low-temperature selective catalytic reduction (SCR) of NOx with NH3. Then, they were subjected to appropriate characterizations for their properties (XRD, TEM, BET, XPS, etc.). The catalytic activity of MnFeTiOx/ATP catalyst was over 95% NOx conversion within a wide temperature window between of 175 and 300 °C, and 88% N2 selectivity. Moreover, MnFeTiOx/ATP presented excellent potassium resistance relative to the traditional V–W–Ti catalyst, and its denitration performance was significantly improved. The NOx conversion rate could be restored to nearly 90% at 210 °C after removing potassium via washing of K–MnFeTiOx/ATP. In addition, the MnFeTiOx/ATP showed better SO2 resistance and stability than the traditional V–W–Ti catalyst. Therefore, the MnFeTiOx/ATP catalyst has been proved to have broad prospects in NH3-SCR.
- Type
- Article
- Information
- Journal of Materials Research , Volume 34 , Issue 7: Focus Section: Interconnects and Interfaces in Energy Conversion Materials , 15 April 2019 , pp. 1188 - 1199
- Copyright
- Copyright © Materials Research Society 2019
Footnotes
These authors contributed equally to this work.
References
- 10
- Cited by




