Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Jansen, S.A.
Grabania, S.A.
Buecheler, N.M.
and
Whitwell, G.
1992.
Electronic Properties of Alumxna/Titania Mixed Oxides and Interfacial Surface Models: a Theoretical Analysis.
MRS Proceedings,
Vol. 291,
Issue. ,
Foster, C. M.
Chan, S.-K.
Chang, H.L.M.
Chiarello, R. P.
and
Lam, D. J.
1993.
Prism-Film Coupling in Anisotropic Planar Waveguides of Epitaxial (101) Rutile Thin Films.
MRS Proceedings,
Vol. 310,
Issue. ,
Ritala, Mikko
Leskelä, Markku
Nykänen, Erja
Soininen, Pekka
and
Niinistö, Lauri
1993.
Growth of titanium dioxide thin films by atomic layer epitaxy.
Thin Solid Films,
Vol. 225,
Issue. 1-2,
p.
288.
Foster, C. M.
Chan, S.-K.
Chang, H. L. M.
Chiarello, R. P.
Zhang, T. J.
Guo, J.
and
Lam, D. J.
1993.
Electromagnetic modes and prism-film coupling in anisotropic planar waveguides of epitaxial (101) rutile thin films.
Journal of Applied Physics,
Vol. 73,
Issue. 11,
p.
7823.
Shin, H.
Collins, R.J.
De Guire, M.R.
Heuer, A.H.
and
Sukenik, C.N.
1995.
Synthesis and characterization of TiO2 thin films on organic self-assembled monolayers: Part I. Film formation from aqueous solutions.
Journal of Materials Research,
Vol. 10,
Issue. 3,
p.
692.
Lee, C.H.
and
Ching, C.W.
1997.
MOCVD TiO2 thin-film doping with microwave PECVD TiC and ZrO2.
Materials Chemistry and Physics,
Vol. 47,
Issue. 2-3,
p.
193.
Gao, Y
Thevuthasan, S
McCready, D.E
and
Engelhard, M
2000.
MOCVD growth and structure of Nb- and V-doped TiO2 films on sapphire.
Journal of Crystal Growth,
Vol. 212,
Issue. 1-2,
p.
178.
Yamamoto, S
Sumita, T
Sugiharuto
Miyashita, A
and
Naramoto, H
2001.
Preparation of epitaxial TiO2 films by pulsed laser deposition technique.
Thin Solid Films,
Vol. 401,
Issue. 1-2,
p.
88.
Cosandey, F.
Zhang, L.
and
Madey, T.E.
2001.
Effect of substrate temperature on the epitaxial growth of Au on TiO2(110).
Surface Science,
Vol. 474,
Issue. 1-3,
p.
1.
Bernardi, M. I. B.
Lee, E. J. H.
Lisboa-Filho, P. N.
Leite, E. R.
Longo, E.
and
Souza, A. G.
2002.
Influence of the growth parameters on TiO2 thin films deposited using the MOCVD method.
Cerâmica,
Vol. 48,
Issue. 308,
p.
192.
Zhu, M
Chikyow, T
Ahmet, P
Naruke, T
Murakami, M
Matsumoto, Y
and
Koinuma, H
2003.
A high-resolution transmission electron microscopy investigation of the microstructure of TiO2 anatase film deposited on LaAlO3 and SrTiO3 substrates by laser ablation.
Thin Solid Films,
Vol. 441,
Issue. 1-2,
p.
140.
Doucette, L.D.
Christensen, T.M.
DeSisto, W.J.
and
Lad, R.J.
2006.
CrO2 (100) and TiO2 (100) film heteroepitaxy on a BaF2 (111)/Si (100) substrate.
Journal of Crystal Growth,
Vol. 290,
Issue. 2,
p.
653.
Chambers, Scott A.
2010.
Epitaxial Growth and Properties of Doped Transition Metal and Complex Oxide Films.
Advanced Materials,
Vol. 22,
Issue. 2,
p.
219.
Wu, Qi-Hui
2011.
The Atomic Structure of Oxide/Oxide Interface.
Critical Reviews in Solid State and Materials Sciences,
Vol. 36,
Issue. 1,
p.
1.
Kim, Dai Hong
Kwon, Ji-Hwan
Kim, Miyoung
and
Hong, Seong-Hyeon
2011.
Structural characteristics of epitaxial SnO2 films deposited on a- and m-cut sapphire by ALD.
Journal of Crystal Growth,
Vol. 322,
Issue. 1,
p.
33.
Wang, Chaojun
Dho, Joonghoe
and
Geul Lee, Sang
2013.
Pseudo-hexagonal in-plane alignment of rutile (100)Nb:TiO2 on hexagonal (0001)Al2O3 plane.
Journal of Crystal Growth,
Vol. 380,
Issue. ,
p.
118.
Lu, Yang
Chiang, Ching-Yu
Li, Yao
Ku, Ching-Shun
Yan, Hao
Huang, Eugene
Chen, Bin
and
Tamura, Nobumichi
2021.
Twinning-mediated anomalous alignment of rutile films revealed by synchrotron X-ray nanodiffraction.
iScience,
Vol. 24,
Issue. 4,
p.
102278.
Pan, Chenggang
Ren, Hongxin
He, Peng
Shi, Ji
Tang, Bin
Yang, Hui
Gao, Changhua
Yang, Fei
Wan, Lichao
and
Xu, Ziqi
2024.
In-situ synthesis and oxidation behaviors of Ti-xAl coatings by high-frequency induction heated combustion.
Journal of Alloys and Compounds,
Vol. 988,
Issue. ,
p.
174232.
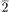 0) α−Al2O3
0) α−Al2O3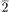 0) sapphire (α−Al2O3) by the MOCVD technique have been characterized by transmission electron microscopy (TEM) and high resolution electron microscopy (HREM). The TiO2 rutile thin films grew on the sapphire with two epitaxial orientations. The epitaxial orientation relationships between the rutile films (R) and the sapphire substrate (S) were found to be (1) (101)[010]R ‖ (11
0) sapphire (α−Al2O3) by the MOCVD technique have been characterized by transmission electron microscopy (TEM) and high resolution electron microscopy (HREM). The TiO2 rutile thin films grew on the sapphire with two epitaxial orientations. The epitaxial orientation relationships between the rutile films (R) and the sapphire substrate (S) were found to be (1) (101)[010]R ‖ (11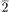 0)[0001]s. Detailed atomic structures of near-interface regions have been investigated by HREM, providing a clear picture of the initial stage of film growth. HREM images show that about 70% of the nuclei at the interface are the (101) rutile, but most of them are very small, about 5 nm (or 2% of the film thickness) in the growth direction. The film growth was dominated by the (200) orientation. Nucleation and growth of the films will be discussed in terms of the lattice mismatch at the interface and growth rates along the two orientations. Planar defects such as twin boundaries and special grain boundaries are commonly observed in the films, especially in regions close to the substrate. The twin plane and twinning direction are {101} and 〈101〉, respectively. Special grain boundaries are found to be correlated with nucleation and twinning.
0)[0001]s. Detailed atomic structures of near-interface regions have been investigated by HREM, providing a clear picture of the initial stage of film growth. HREM images show that about 70% of the nuclei at the interface are the (101) rutile, but most of them are very small, about 5 nm (or 2% of the film thickness) in the growth direction. The film growth was dominated by the (200) orientation. Nucleation and growth of the films will be discussed in terms of the lattice mismatch at the interface and growth rates along the two orientations. Planar defects such as twin boundaries and special grain boundaries are commonly observed in the films, especially in regions close to the substrate. The twin plane and twinning direction are {101} and 〈101〉, respectively. Special grain boundaries are found to be correlated with nucleation and twinning.



