Article contents
Metallic glass-forming composition range of the Cu–Zr–Ti ternary system determined by molecular dynamics simulations with many-body potentials
Published online by Cambridge University Press: 22 February 2011
Abstract
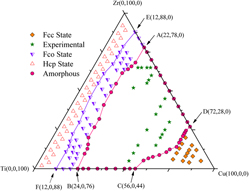
An n-body Cu–Zr–Ti potential is constructed and applied to evaluate a glass-forming composition range (GFR) of the Cu–Zr–Ti ternary system by molecular dynamics simulations using a solid-solution model, which is formed via random substitution of solvent atoms by a certain number of solute atoms. It is found that the GFR of the Cu–Zr–Ti ternary system is located within an approximate distorted quadrilateral composition region, in which the solid solutions are unstable and spontaneously collapse to form amorphous phases. The compositions of the four vertexes of the distorted quadrilateral are determined to be Cu22Zr78Ti0, Cu24Zr0Ti76, Cu56Zr0Ti44, and Cu72Zr28Ti0, respectively. In addition, the simulation results are in good agreement with the experimental observations and compatible with some empirical rules.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 7
- Cited by




