Article contents
The long-term corrosion of mild steel in depassivated concrete: Localizing the oxygen reduction sites in corrosion products by isotopic tracer method
Published online by Cambridge University Press: 12 December 2011
Abstract
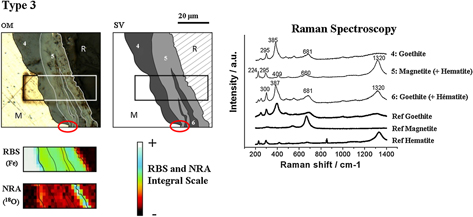
Over a long period (>10 years), the prediction of iron/mild steel corrosion in concrete requires the use of a mechanistic approach. For that purpose, a key point of the mechanisms involved is the localization of the oxygen reduction sites within the thick corrosion layers, which may greatly influence the nature of the rate-limiting step. In this context, iron rebars (originally covered with concrete) were sampled from a 50-year-old historical building and submitted to isotopic tracers methods (18O) combined with structural Raman microspectroscopy analyses on transverse sections. By this method, the authors demonstrate that the oxygen reduction sites are strongly impacted by the presence of a conductive phase (magnetite) in contact with the metallic substrate.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 7
- Cited by




