Article contents
Intermetallic compound spalling characteristics of Sn-3.5Ag solder over ternary electroless Ni under-bump metallurgy
Published online by Cambridge University Press: 17 November 2011
Abstract
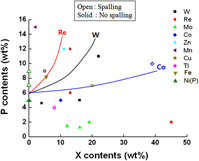
Ternary electroless nickel, NiXP, films were produced by adding salts of Mo, Re, Tl, Cu, W, Co, Fe, Zn, and Mn to conventional electroless Ni baths and subsequently reacted with Sn-3.5Ag solder. From the full width at the half maximum (FWHM) data, as-plated NiXP films can be categorized into two groups: one is close to the FWHM value of nanocrystalline Ni5P film and the other is close to amorphous Ni9P film. Alloying elements in the electrolessly plated under-bump metallurgy that effectively suppressed intermetallic compound (IMC) spalling were Mn, Zn, Re, Fe, and W, whereas Tl exacerbated spalling. The roles of Cu, Mo, and Co were less clear due to a lack of data. Based on scanning electron microscopy observations, a spalling map was presented, which showed elemental demarcation lines of IMC spalling in the X-P coordinates.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 1
- Cited by




