Article contents
Fabrication and deformation of aluminum–manganese microsandwich structure
Published online by Cambridge University Press: 11 February 2016
Abstract
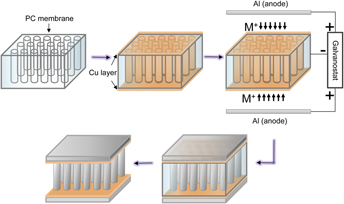
The combination of low areal density, high flexural rigidity, and open architecture makes metallic microsandwiching a promising candidate for structural frameworks in small-scale multifunctional devices. We demonstrate a one-step electrodeposition procedure to synthesize an aluminum–manganese (Al–Mn) microsandwich using a porous polycarbonate (PC) membrane template from room-temperature ionic liquid. Mn was added to refine the microstructure and increase the hardness of Al. A cyclic voltammogram study shows Mn codeposit with Al in an acidic chloroaluminate electrolyte. Increasing the MnCl2 concentration in the electrolyte from 0.05 to 0.25 M promoted a crystalline to amorphous phase transition of the deposited structures. Finally, mechanical properties and damage resistance of the microsandwiches were evaluated using nano- and micro-indentation tests as well as finite element methods.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 2
- Cited by





