Article contents
Enhancement on afterglow properties of Eu3+ by Ti4+, Mg2+ incorporation in CaWO4 matrix
Published online by Cambridge University Press: 07 February 2012
Abstract
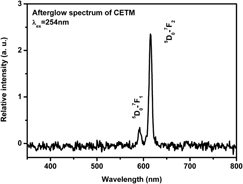
The long afterglow phosphor, CaWO4: Eu3+, is synthesized and the intensity and duration of its afterglow can be enhanced by the Ti4+ and Mg2+ incorporation. The x-ray diffraction patterns depict pure tetragonal CaWO4 of all samples. The emission spectra show the Eu3+ emission and the charge transfer (CT) emission of WO42−. The intensity of CT increases with the Mg2+ incorporation. The excitation spectra monitoring 616 nm exhibit the strongest CT band with Ti4+ incorporation. These results indicate that Mg2+ enhances the efficiency of CT emission of WO42− while the Ti4+ enhances the energy transfer rate from CT to Eu3+. Since the thermoluminescence (TL) curves do not imply a new trap, the enhancement of the afterglow results from the coreinforcement of CT efficiency and energy transfer rate.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 7
- Cited by




