Article contents
Characterization of structure and properties of polymer films made from blends of polyethylene with poly(4-methyl-1-pentene)
Published online by Cambridge University Press: 22 December 2016
Abstract
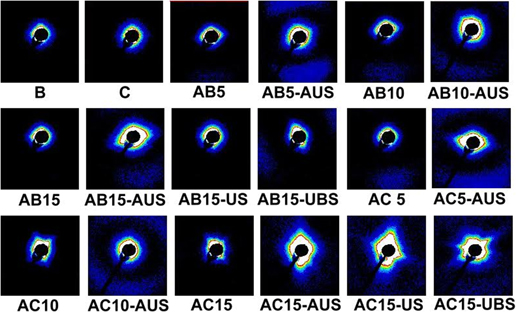
The main aim of this research was investigation of the processing-structure-property relationship for polymer blends. The paper presents the results of tests on the structure, basic physical and porous properties of polymer films blend of low density polyethylene (LDPE) with poly(4-methyl-1-pentene) (PMP). Studies utilizing LDPE/PMP blends were undertaken to investigate a three-stage process: melt-extrusion/annealing/uniaxial-stretching (MEAUS), and a two-stage process: melt-extrusion/uniaxial and biaxial stretching (MEUS and MEUBS), used to produce porous films. The permeability and porosity results coupled with small-angle x-ray scattering data provide a direct connection between changes in microstructure to the observed changes in gas transport properties.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 7
- Cited by





