Article contents
Asparagine–serine–serine peptide regulates enamel remineralization in vitro
Published online by Cambridge University Press: 22 October 2013
Abstract
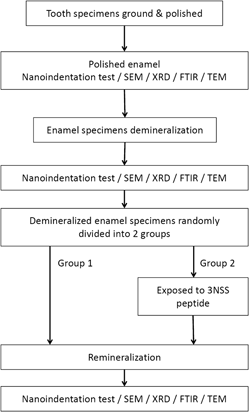
A highly biocompatible peptide, triplet repeats of asparagine–serine–serine (3NSS) regulates mineral deposition for the reconstruction of erosive enamel. Healthy human enamel was demineralized to create lesions, then exposed to the 3NSS peptide solution, and finally immersed in artificial saliva. The degrees of nanohardness recovery were 5.02% and 16.27% for the control group and enamel treated with the 3NSS peptide, respectively. Peptides assembling at enamel interrod attracted greater quantities of ions from the solution to form nanocrystalline hydroxyapatite minerals during the reconstruction of vacant gap. This resulted in a decrease in the surface roughness, and the acidic eroded pores were filled completely. Additionally, the newly deposited hydroxyapatites remineralized with the aid of the 3NSS peptide exhibited a smaller average crystalline size, which effectively inhibited plastic deformations. Treatment with the 3NSS peptide provided great improvements in nanohardness and elastic modulus.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 5
- Cited by




