No CrossRef data available.
Article contents
Analysis of effects of reactant particle size on phase transformations in the Ti–Si–Cu system using differential thermal analysis and x-ray diffraction
Published online by Cambridge University Press: 03 July 2012
Abstract
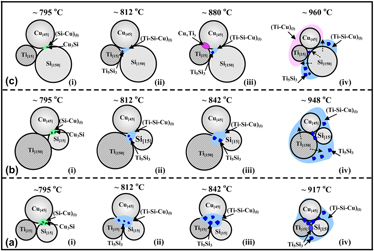
Effects of Ti and Si particle sizes on phase transformations of Ti–Si–Cu system were explored through differential thermal analysis (DTA), x-ray diffraction (XRD), and field emission scanning electron microscope (FESEM). For Ti[15]Si[15]Cu[45] system, fine Ti easily dissolves into Si–Cu liquid to form Ti–Si–Cu liquid at ∼795 °C, which further participates into the reaction of β-Ti and Si to yield abundant quantity of Ti5Si3 at ∼917 °C. For Ti[150]Si[15]Cu[45] system, nonetheless, the reaction of coarse Ti with Si–Cu liquid involves more difficulty in forming the ternary liquid , which is the causal factor for the delay in the formation of Ti5Si3 to ∼948 °C. For Ti[15]Si[150]Cu[45] system, coarse Si results in the formation of insufficient Si–Cu liquid initially, whereas Ti–Cu liquid forms at ∼960 °C instead, which further reacts with coarse Si to form Ti–Si–Cu liquid, and then Ti5Si3is precipitated from the liquid.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012




