Article contents
Submicro-sized Si–Ge solid solutions with high capacity and long cyclability for lithium-ion batteries
Published online by Cambridge University Press: 25 May 2018
Abstract
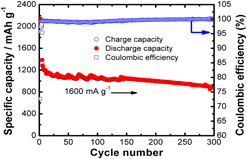
Mastery of strengthening strategies to achieve high-capacity anodes for lithium-ion batteries can shed light on understanding the nature of diffusion-induced stress and offer an approach to use submicro-sized materials with an ultrahigh capacity for large-scale batteries. Here, we report solute strengthening in a series of silicon (Si)–germanium (Ge) alloys. When the larger solute atom (Ge) is added to the solvent atoms (Si), a compressive stress is generated in the vicinity of Ge atoms. This local stress field interacts with resident dislocations and subsequently impedes their motion to increase the yield stress in the alloys. The addition of Ge into Si substantially improves the capacity retention, particularly in Si0.50Ge0.50, aligning with literature reports that the Si/Ge alloy showed a maximum yield stress in Si0.50Ge0.50. In situ X-ray diffraction studies on the Si0.50Ge0.50 electrode show that the phase change undergoes three subsequent steps during the lithiation process: removal of surface oxide layer, formation of cluster-size Lix(Si,Ge), and formation of crystalline Li15(Si,Ge)4. Furthermore, the lithiation process starts from higher index facets, i.e., (220) and (311), then through the low index facet (111), suggesting the orientation-dependence of the lithiation process in the Si0.50Ge0.50 electrode.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
Footnotes
These authors contributed equally to this work.
References
REFERENCES
- 11
- Cited by





