Article contents
Nitrite sensor based on room temperature ionic liquid functionalized α-zirconium phosphate modified glassy carbon electrode
Published online by Cambridge University Press: 24 August 2020
Abstract
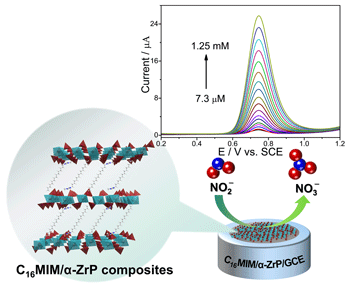
A novel ionic liquid/α-ZrP (C16MIM/α-ZrP) lamellar nanocomposite was fabricated via the electrostatic self-assembly deposition technique by using exfoliated α-ZrP nanosheets and guest molecules (1-hexadecyl-3-methylimidazolium bromide) as building blocks under mild conditions. C16MIM/α-ZrP nanocomposite was characterized by various analytical techniques such as X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscope (SEM), Fourier transform infrared spectroscopy, and synchronous thermal analyzer. The net interlayer spacing of α-ZrP determined by XRD confirmed that the C16MIM cations formed a monolayer arrangement between the α-ZrP nanosheets. The morphology and microstructure of C16MIM/α-ZrP composite were observed using SEM and TEM. The C16MIM/α-ZrP modified glass carbon electrode exhibited excellent electrocatalytic activity toward the oxidation of nitrite in weak base media. The results obtained with differential pulse voltammetry demonstrated that the C16MIM/α-ZrP hybrid detected nitrite linearly in the concentration range from 7.3 μM to 1.25 mM with the detection limit of 1.26 μM (S/N = 3). Additionally, the prepared sensor showed outstanding reproducibility, high stability, and anti-interference capability.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2020, published on behalf of Materials Research Society by Cambridge University Press
References
- 4
- Cited by



