Article contents
EPR and optical properties of KY(WO4)2:Gd3+ powders
Published online by Cambridge University Press: 12 November 2012
Abstract
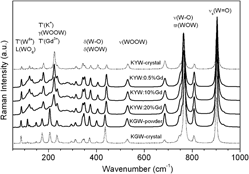
Comparisons of structural, optical, and magnetic properties between KY(WO4)2 (KYW) powders doped with Gd3+ from 0.5 up to 100 mol% and KGd(WO4)2and KYW single crystals have been made. For this purpose, x-ray diffraction (XRD), infrared (IR), Raman, and electron paramagnetic resonance (EPR) spectra were collected. The XRD studies have verified the quality of the synthesis of compounds and have shown the differences in the positions of the diffraction peaks due to the change in concentration of gadolinium ions. Raman and IR spectra confirmed that the phases are isostructural. The optimization of the spin Hamiltonian parameters and EPR data simulation was achieved by using the electron paramagnetic resonance and nuclear magnetic resonance (EPR-NMR) program. Changes in kind of magnetic interactions were found and analyzed from the point of view of their dependence of the compound form (powder, single crystal), temperature, and gadolinium ion concentration. The investigated compounds revealed complex interactions between gadolinium ions both in a type and a strength.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 5
- Cited by




