Introduction
Natural selection should favour parasites that manipulate their host in ways that increase parasite fitness, and there are now many examples of adaptive parasitic manipulation across a wide range of parasite taxa (Moore, Reference Moore1984; Berdoy et al., Reference Berdoy, Webster and Macdonald2000; Barber et al., Reference Barber, Walker and Svensson2004; Yanoviak et al., Reference Yanoviak, Kaspari, Dudley and Poinar2008; Wesołowska & Wesołowski, Reference Wesołowska and Wesołowski2014; Westwood et al., Reference Westwood, O'Donnell, de Bekker, Lively, Zuk and Reece2019; Lovett et al., Reference Lovett, St. Leger and de Fine Licht2020). However, it can sometimes be difficult to determine whether parasite-induced changes are adaptive, or whether the changes are simply byproducts of infection (Poulin, 1995, Reference Poulin2010). In addition, the effects of infection can be complex. For example, Lagrue et al. (Reference Lagrue, McEwan, Poulin and Keeney2007) found that field-collected snails (Potamopyrgus antipodarum) infected by a trematode (Coitocaecum parvum) were smaller (by volume) than uninfected snails; but they also found that the internal shell volume available for parasite reproduction represented a larger proportion of the total internal shell volume. Based on these results, they suggested that the parasite might manipulate infected snails to increase the relative ‘parasitized’ shell volume of their hosts, even though infection reduces overall shell size (perhaps due to the energetic costs of infection). In other words, there may be adaptive parasitic manipulation nested within non-adaptive constraints. In the present study, we experimentally evaluated the effect of infection by a different digenetic trematode (Atriophallophorus winterbourni) on the growth and shell morphology of P. antipodarum.
Potamopyrgus antipodarum is a freshwater snail native to New Zealand. This species of snail is infected by at least a dozen species of helminth parasites, of which the trematode A. winterbourni is the most common in lake populations (Winterbourn, Reference Winterbourn1973; Lively, Reference Lively1987; Vergara et al., Reference Vergara, Lively, King and Jokela2013). Infections by this long-studied, but newly described, trematode (Blasco-Costa et al., Reference Blasco-Costa, Seppälä, Feijen, Zajac, Klappert and Jokela2019) were found to induce risky foraging behaviour in the snail, which likely increases the probability of parasite transmission to the definitive host, waterfowl (Levri & Lively, Reference Levri and Lively1996). In a follow-up study, Levri et al. (Reference Levri, Dillard and Martin2005) found that the shells of infected snails tended to be longer, and with a greater width-to-length ratio. Given that the parasite encysts inside the shell, and that the internal volume of the shell determines the number of cysts produced, the authors suggested that parasites might adaptively modify snail growth to increase the internal volume. However, as Levri et al. (Reference Levri, Dillard and Martin2005) clearly state, alternative hypotheses are possible, especially given that the snails were collected in the field and represented multiple age classes. It is possible, for example, that older (and larger) snails are more likely to be infected, because the cumulative probability of exposure to parasite eggs increases over time (Jokela & Lively, Reference Jokela and Lively1995; Levri et al., Reference Levri, Dillard and Martin2005). In the present study, we took an experimental approach to determine whether infection (or exposure to infection) altered the developmental morphology of juvenile snails.
Methods
Model organisms
Potamopyrgus antipodarum is a small (<6 mm) freshwater snail that is widely distributed in lakes, rivers and streams throughout New Zealand. Some populations are almost entirely composed of polyploid asexual females, while other populations contain a mixture of polyploid asexual females and diploid sexual individuals. In the sexual fraction of the population, the sexes are separate (males and females). Embryos develop in a brood pouch and ‘crawl out’ of their mother's shell (ovoviviparity) in both sexual and asexual females. The pouch is located in the first whorl of female shells, and the width of this whorl is larger in females compared to males in our study population at Lake Alexandrina (Levri et al., Reference Levri, Dillard and Martin2005), which would allow for a great number of embryos in the brood pouch (see also Jakubik, Reference Jakubik2006).
Atriophallophorus winterbourni is the most common trematode parasite of P. antipodarum in New Zealand lakes (Lively, Reference Lively1987; Vergara et al., Reference Vergara, Jokela and Lively2014). This trematode (previously called Microphallus sp.) has a two-host life cycle. The hermaphroditic adult worms live in the intestines of ducks, where they cross-fertilize and produce eggs. The eggs are shed into the environment in the faeces of infected waterfowl, primarily ducks and New Zealand scaup (Osnas & Lively, Reference Osnas and Lively2011). The eggs hatch after being ingested by snails, and the larval miracidia infect the snail's viscera. The larvae reproduce asexually as geminal balls (Hechinger, Reference Hechinger2012) eventually replacing the host's reproductive tissues, leading to complete sterilization of the snail. The geminal balls develop, eventually encysting as metacercariae before becoming competent for transmission to the final avian host. The fully developed metacercariae are detectable by 3–4 months post-exposure under laboratory conditions (Lively & McKenzie, Reference Lively and McKenzie1991). The life cycle is completed when infected snails are ingested by waterfowl. It is important to note that the visceral volume of infected snails places an upper limit on the number of metacercaria produced, and that the region is normally packed with metacercariae in transmission-ready infections (C. Lively, personal observations; K. Klappert and J. Jokela, unpublished data). Hence, selection might favour parasites that manipulate growth in the snails to increase the visceral volume.
Mesocosm experiment
On 8 and 9 February 2016, P. antipodarum snails were collected from the shallow-water margins of Lake Alexandrina (McKenzie Basin, South Island, New Zealand). The snails were collected from 14 different sites around the lake by pushing a kick net through the submerged roots of willow trees. Snails in this shallow region of the lake are predominately diploid sexuals (Vergara et al., Reference Vergara, Lively, King and Jokela2013), but the region also contains a diverse clonal population {Fox et al., Reference Fox, Dybdahl, Jokela and Lively1996, 98; Million et al., Reference Million, Bhattacharya, Dinges, Montgomery, Smith and Lively2021, 2916; Paczesniak et al., Reference Paczesniak, Adolfsson, Liljeroos, Klappert, Lively and Jokela2014, 2219}, which shows rapid turnover in clone frequencies, most likely driven by parasite-mediated frequency-dependent selection {Paczesniak, Reference Paczesniak, Adolfsson, Liljeroos, Klappert, Lively and Jokela2014, 2219}. The frequency of infection also oscillates over time such that the sexual diploids are more or less infected than the polyploid asexuals (Gibson et al., Reference Gibson, Delph, Vergara and Lively2018). Diploid snails were more infected than triploid snails during 2016 when the samples for the present study were collected (Gibson et al., Reference Gibson, Delph, Vergara and Lively2018). We also collected duck faeces, which contain large numbers of A. winterbourni eggs, from the banks of Lake Alexandrina at two different sites. The snails and faeces were then transported in coolers containing frozen refrigerant blocks to the Edward Percival Field Station (run by the University of Canterbury) in Kaikoura, New Zealand. At the field station, the snails from the 14 different sites were placed into separate 20-l plastic trays and fed ad libitum on spirulina powder. The water was changed every second day. The faeces were placed in 20-l plastic containers, for which the water was changed several times per day to remove soluble waste. The faecal slurry was then sieved through a kitchen sieve (1.5 mm) to remove large particles.
After several days, the snails were sieved to separate juveniles (<2.5 mm length) from adults. Approximately 220 juveniles from each of the 14 sites were combined into a single mixture of snails (N > 3000 total snails). Juvenile snails were then randomly selected from this mixture until we had 20 groups of 100 snails per group (N = 2000). These 20 groups of snails were then placed into separate 2-l plastic containers. Ten of these containers were then randomly selected for the addition of parasite eggs from the faecal slurry; the snails in the other 10 containers (controls) were not exposed to the slurry. Prior to parasite exposure on 19 February 2016, we combined the faecal samples from both sites, and we carefully decanted the water until the volume of the mixture was one litre. The experimental containers were then given 15 ml each (5 shots of 3 ml each) of slurry, which based on previous experiments, is usually sufficient to reach moderate levels of infection. The controls were given a mixture of water and spirulina powder. The controls allowed us to estimate the level of background infection from exposures in the field prior to collection.
After three days of experimental exposure to parasite eggs, we transferred the snails from each of the 20 containers to separate 2-l mesh cages constructed from 250-μ nylon mesh. The bags were held open by 2-l plastic soda bottles in which the sides and bottoms were cut out, leaving only a plastic frame (see fig. 1 in Paczesniak et al. (Reference Paczesniak, Klappert, Kopp, Neiman, Seppälä, Lively and Jokela2019) for an illustration of a smaller version of the cages). The bags were then placed in a single, 800-l outdoor mesocosm at the Edward Percival Field Station in Kaikoura, New Zealand. The mesocosm was then covered with a mesh shade cloth. The snails were left in the mesocosm to develop for about 11 months.
On 24 January 2017, the snails were removed from the nylon mesh cages, and the experimental and controls’ snails were separately combined in 20-l containers. Survivorship in the cages was very high, but one of the experimental cages was lost in the processing. The snails were then fed ad libitum, and the water was changed every 2–3 days. The snails were then transported to Indiana University, USA, for morphological analysis on 13 February 2017.
Dissection and morphological analysis
A total of 181 snails were randomly selected for dissection and morphometric analysis, of which 92 had been exposed to parasite eggs and 89 were from controls. For the morphometric analyses, the shell was placed on a dry surface with the aperture facing up. A digital photograph of each snail was taken using a Sony Cybershot HD camera 720p. These photographs were used to measure shell dimensions using Image J (see fig. 1). Each snail was then dissected to determine its sex and brooding condition, and then scored for the presence of infection. Snails infected with parasite species other than A. winterbourni (e.g., Notocotylus, N = 2) were uncommon and excluded from the analysis. The photographs were used to analyse shell morphology using Image J. Note that a previous morphological study of this snail examined shell-size and shell-shape variation among field-collected animals from 17 different lake populations (Vergara et al., Reference Vergara, Fuentes, Stoy and Lively2017). The study showed that shell shape and size were not significantly associated with the prevalence of infection or the frequency of males in the population, but it did not contain individual-level data for sex and infection.

Fig. 1. Drawing of a snail showing the measurements used to assess shell size and internal volume. Coloured arrows correspond to width measurement: (1) total width; and (2) visceral width.
As indicated above, A. winterbourni lives and reproduces in the gonads and digestive glands of the snails in the posterior part of the shell (from the second whorl to the tip of the shell, which contains the viscera). Hence, following Lagrue et al. (Reference Lagrue, McEwan, Poulin and Keeney2007), we measured ‘visceral length’ as the distance from the second whorl to the tip of the shell, and the ‘visceral width’ as the width of second whorl (Lagrue et al. (2007) called these measures ‘parasitized length’ and ‘parasitized width,’ respectively). We also measured total shell width as the width of the first whorl, and we measured total shell length as the total length of the shell. Also following Lagrue et al. (Reference Lagrue, McEwan, Poulin and Keeney2007), we calculated the visceral volume of the snails as V = (πr 2h)/3, where V is the visceral volume, r the radius (i.e., the visceral width), and h the height (the visceral length), to give an estimate of the internal volume for packing the metacercariae produced within the snail by A. winterbourni. Lagrue et al. (2007) referred to this measure as the ‘parasitized volume.’
Statistical analysis
All statistical analyses were run in R 4.0.2 (R Core Team, 2020), and confirmed in version 27 of SPSS IBM Corp. (2020). A one-way analysis of variance (ANOVA) on uninfected females showed that there was no significant effect of exposure per se on either shell length (F1, 94 = 0.178, P = 0.674) or visceral length (F1, 94 = 1.187, P = 0.279), so we combined the uninfected females from the exposed and control treatments in our analyses. Similar results were observed when comparing uninfected males in exposures vs. controls for both shell length (F1, 40 = 1.014, P = 0.32) and visceral length (F1, 40 = 2.747, P = 0.105), thus uninfected males from both treatments were combined for analysis. In these subsequent analyses, we compared five different groups: (1) brooding females (all brooding females are uninfected); (2) uninfected, non-brooding females; (3) infected females; (4) uninfected males; and (5) infected males. Using the R packages FactoMineR (Le et al., Reference Le, Josse and Husson2008) and facto extra (Kassambara & Mundt, Reference Kassambara and Mundt2020), we first performed a principal component analysis (PCA) on the four shell measurements: total shell length; shell width (width of the widest whorl); visceral length (length of all but the first whorl); and visceral width (width of the second largest whorl, fig. 1). We conducted the PCA to avoid having strongly positively correlated dependent variables (table 1). We then performed a multivariate analysis of variance (MANOVA) using the first two principal components (PCs) to test for differences among groups. ANOVA was then run on each PC, followed by Tukey tests to identify groups that significantly differed. We also used ANOVA to compare the mean visceral volumes for infected and uninfected individuals of similar size to test for adaptive parasitic manipulation.
Table 1. Correlation matrix. Correlations among all measured variables, including collations to principal component 1 (PC1) and principal component 2 (PC2). Values in parentheses give the percentage contributions of each variable to PC1 and PC2. Each variable's contribution would be 25% if the contributions were uniform.

Results
In the exposure treatment, 40 of 92 snails were infected (table 2). By contrast, in the control treatment, only three individuals out of 89 were infected. These results suggest that the level of infection in the field-collected (control) snails was low, and that the experimental exposures to parasite eggs led to successful infections. In exposed snails, the infection frequency was significantly higher in males (0.56) than females (0.34) (χ2 = 4.61; d.f. = 1; P = 0.032) (See table 2). Because males are diploid and diploids were more infected in 2016, this result is consistent with the findings of a long-term field experiment (Gibson et al., Reference Gibson, Delph, Vergara and Lively2018).
Table 2. Sex, infection status and brooding status for individuals in each treatment: exposed to parasite eggs vs. controls.

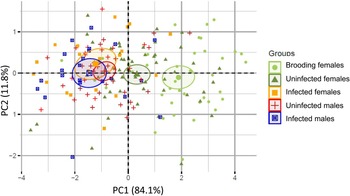
We measured total shell length, visceral length, total shell width and visceral width for all snails (table 3). Principal component 1 (PC1) was positively correlated to all four measures of shell size, and it explained 84.1% of the variance. Principal component 2 (PC2) explained only 11.8% of the total variation. PC2 was negatively related to total shell width, and positively related to visceral length (table 1) and thus give a shape measure, whereas long shells relative to width have positive values, and short shells relative to width have negative values. The first two PCs together explained 95.9% of the variance. None of the other PCs were extracted by the analysis.
Table 3. Mean values (±standard error) for all five snail groups (at the end of the experiment) for each of the measured values (in cm) and the calculated values for visceral volume (in cm3). The juvenile snails at the start of the experiment were approximately 2.0 to 2.5 mm in total length. Uninfected snails were fully mature by the end of the experiment.

The MANOVA using PC1 and PC2 scores as the dependent variables showed highly significant differences among groups (Pillai = 0.451, P ≪ 0.001). Subsequent ANOVAs on each PC showed that the groups were significantly different for PC1 (F4,176 = 33.54, P ≪ 0.001), but not PC2 (F4,176 = 0.83, P = 0.508). A post-hoc Tukey honest significant differences test revealed three homogeneous subsets in the data for PC1. Brooding females were larger than all other groups of snails (P < 0.001) (fig. 2). Non-brooding uninfected female snails were smaller than brooding females, but larger than the remaining three groups (P < 0.001). The third group contained infected females, infected males and uninfected males, wherein there were no significant differences in PC1 among the members of the group. These results therefore indicate that infection stunts the growth of females and renders them to be similar in total size to infected and uninfected males (fig. 2). PC2 did not differ among groups, suggesting that the parasite is not inducing shape changes in the developing snails. Moreover, the visceral volumes of infected males, infected females, and uninfected males were not significantly different (ANOVA, F2,82 = 0.891, P = 0.414), suggesting that, controlling for shell size, parasites are not manipulating the parasitized volume of their hosts. Taken together, these results suggest that infection is decreasing, rather than increasing, the internal shell volume for parasite reproduction in female hosts.

Fig. 2. Principal component analysis (PCA) results for the 198 individuals studied. PCA was based on four morphological traits: total shell length; visceral length; width of the widest whorl; and width of the second widest whorl (or visceral width). Principal component 1 was highly correlated with total length, total width, and visceral width, and it represents overall shell size; and principal component 2 (PC2) was positively correlated with visceral length, but negatively correlated with total width (table 1). Hence PC2 seems to be related to shell shape, where relatively long and thin (high spired) shells have high positive values, and relatively wide shells (low spired) have negative values. Symbols and colours correspond to the five distinct groups of snails: light green circles correspond to uninfected brooding females (N = 41); dark green triangles correspond to uninfected non-brooding females (N = 55); orange squares correspond to infected females (N = 20); red crosses correspond to uninfected males (N = 42); and blue squares with an x correspond to infected males (N = 23). Ellipses correspond to the 95% confidence interval of each group gravity centre. Overlapping ellipses indicate non-significant morphological differences among groups.
Discussion
Parasites should be under selection to manipulate host phenotypes in ways that increase parasite fitness, but it can be difficult to separate adaptive parasitic manipulation from the side effects caused by infection (Poulin, Reference Poulin1995, Reference Poulin2010; Thomas et al., Reference Thomas, Adamo and Moore2005). In the present study, we exposed juvenile snails (P. antipodarum) to the eggs of a common trematode parasite (A. winterbourni) and reared them to adulthood in outdoor, semi-natural mesocosms. The goal was to determine whether infection altered shell growth and shell morphology in ways that would increase the internal shell volume available for parasite reproduction. We also determined whether infection affected sexual dimorphism in the snail.
We found that uninfected P. antipodarum were sexually dimorphic with respect to size. Specifically, uninfected females were larger than uninfected males (fig. 2). Infected snails, however, were not sexually dimorphic: infected males and infected females were very similar in size (PC1), and both infected males and infected females were almost identical in size to uninfected males (fig. 2). Hence infection stunted female growth, but it did not affect male growth. Thus, contrary to our hypothesis, shell growth in infected females reduced, rather than increased, the internal shell volume available for parasite reproduction.
These results are consistent with a previous, short-term experiment conducted in small (11-l) containers under laboratory conditions (Krist & Lively, Reference Krist and Lively1998). That study showed that infection of juvenile snails stunted their growth (measured as shell length). It also showed that infection did not result in the induction of brooding, which is as expected if there were fecundity compensation (Minchella & Loverde, Reference Minchella and Loverde1981). However, the snails were not sexed, so it could not be determined whether infection affected males and females differently. Based on the results of the present study (under more natural conditions), infection only appears to stunt the growth of females, potentially as a consequence of changes in physiology and/or hormones resulting from parasitic castration.
Taken together, these results suggest the larger size of infected snails in field samples (Jokela & Lively, Reference Jokela and Lively1995; Levri et al., Reference Levri, Dillard and Martin2005) is not the result of parasite manipulation to increase the parasitized volume of infected snails. It remains possible, however, that the parasite's optimal strategy is not to increase the parasitized volume, but rather to produce transmission-ready metacercariae at a rapid rate (Hechinger et al., Reference Hechinger, Lafferty, Mancini, Warner and Kuris2009; Hechinger, Reference Hechinger2010). Under this idea, infected juvenile females might have stunted (rather than enhanced) growth as a parasitic strategy to become transmission-ready more rapidly, especially if the risk of snail mortality is high (Hechinger, Reference Hechinger2010). It is also possible that the rate of trematode development depends on local abiotic conditions, such as water temperature. The present study cannot discriminate between these alternatives.
There are nonetheless some surprising findings from our experimental study. For example, we did not find, as expected from field data (Levri et al., Reference Levri, Dillard and Martin2005), that females were wider per unit length than males. It may be the case that the conditions in our outdoor experimental mesocosm did not permit the normal development of females that are seen under more natural field conditions. If so, the mesocosms may have also prevented the normal development of infected females. Another possibility is that there is morphological variation among clonal genotypes, and that clonal turnover (Paczesniak et al., Reference Paczesniak, Adolfsson, Liljeroos, Klappert, Lively and Jokela2014) changes the average female shell shape over time. Morphological variation among clones might also explain at least some of the variance in PC2. These issues remain as potential questions for future studies.
Finally, we found that brooding females were significantly larger that uninfected non-brooding females. This finding suggests that females experience a growth spurt prior to initiating reproduction. Consistent with this idea, field studies showed that non-brooding females foraged more than brooding females (Levri & Lively, Reference Levri and Lively1996). It is possible that such a growth spurt is driven by an increase in the foraging rate of maturing females, which would likely increase their rate of contact with A. winterbourni eggs in the wild. Feeding rate is associated, for example, with the risk of infection in Daphnia in which fungal spores are ingested as a consequence of filter feeding (Hall et al., Reference Hall, Sivars-Becker, Becker, Duffy and Tessier2007). In addition, larger (older) snails may have simply had more time for exposure to parasite eggs. These ideas might explain why larger snails are more infected in the field, as observed by Levri et al. (Reference Levri, Dillard and Martin2005). Nonetheless it would appear, based on our present results, that the larger size of infected, field-collected snails is not a consequence of adaptive parasitic manipulation.
Acknowledgements
We thank Ed Levri, Amy Krist, David Polly, Maurine Neiman, Zoe Dinges, and Daniela Vergara for helpful comments. We especially thank Ryan Hechinger for a helpful critical review.
Financial support
The fieldwork was funded by the Indiana University Faculty Research Support Program. The analysis and writing phases were supported by the United States National Science Foundation (DEB 1906465).
Conflicts of interest
None.