No CrossRef data available.
Article contents
Evaluation of the role of lisdexamfetamine on attention-deficit/hyperactivity disorder common psychiatric comorbidities: mechanistic insights on binge eating disorder and depression
Published online by Cambridge University Press: 01 September 2022
Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a psychiatric condition in which children suffer from inattentiveness, hyperactivity, and or impulsivity. ADHD patients frequently present comorbid psychiatric disorders: in adults, the most common are depression, substance-related disorders, anxiety, and eating disorders. Children and adolescents present conduct disorders, learning disorders, anxiety and depression. Since ADHD and its psychiatric comorbidities share similarities, a partial overlap of their pathophysiological mechanisms has been suggested. ADHD, can be treated with lisdexamfetamine (LDX), a prodrug indicated by the FDA as treatment for binge eating disorder (BED) and ADHD.
To evaluate, through a systems biology-based in silico method, the efficacy of LDX as first-line ADHD treatment to improve ADHD psychiatric comorbidities. Furthermore, we explored the molecular mechanisms behind LDX’s action.
We used the systems biology- and artificial intelligence-based Therapeutic Performance Mapping System (TPMS) technology to characterise and model ADHD comorbidities. Artificial neural networks (ANNs) algorithms were used to identify specific relationships between protein sets. Finally, we modelled the mechanisms of LDX for the most relevant comorbidities by using sampling methods and comorbidity-specific virtual patients in each case.
This study predicts a strong relationship between LDX’s targets and proteins involved in BED and depression (Fig 1). Our results could be explained not only by LDX role in neurotransmitter regulation, but also by modulation of neuroplasticity (BDNF/NTRK2, GSK3), neuroinflammation (interleukins, inflammasome), oxidative stress (NOS2, SOD), and the hypothalamic-pituitary-adrenal (HPA) axis (CRH, CRHR1).
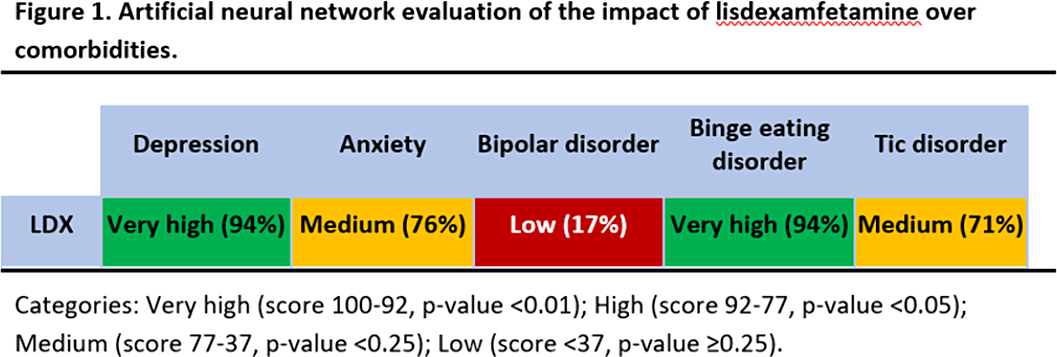
These findings could be used in pre-clinical and clinical future investigations to assess optimal treatment for ADHD patients with psychiatric comorbidities.
JRGC: speaker for Takeda and Shire, research funding from Shire and Lumbeck, collaborations with Laboratoires Servier JQ: speaker or scientific advisor for Takeda, Janssen, Rubio. Investigation funding: Instituto de Salud Carlos III. PR, CM, TPR: full-ti
Keywords
- Type
- Abstract
- Information
- European Psychiatry , Volume 65 , Special Issue S1: Abstracts of the 30th European Congress of Psychiatry , June 2022 , pp. S288 - S289
- Creative Commons
- This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Copyright
- © The Author(s), 2022. Published by Cambridge University Press on behalf of the European Psychiatric Association





Comments
No Comments have been published for this article.