1. Introduction
A wealth of evidence indicates that adverse experiences in early life can predispose to metabolic syndrome and diabetes in adulthood [Reference Campbell, Farmer, Nguyen-Rodriguez, Walker and Egede1], as well as to depressive disorders [Reference Chapman, Whitfield, Felitti, Dube, Edwards and Anda2]. Depression and metabolic impairment are themselves interrelated [Reference Mezuk, Eaton, Albrecht and Golden3], suggesting that childhood adversity may trigger a complex cascade of physiological events that interact continuously over the lifespan to alter both physical and mental health [Reference Danese, Moffitt, Harrington, Milne, Polanczyk and Pariante4].
Less is known about the ways in which a history of early life adversity may affect the body’s response to existing insulin sensitizing treatments. Individuals with diabetes vary in their responsiveness to available antidiabetic treatments, even when treatments are adhered to appropriately [Reference Manolopoulos, Ragia and Tavridou5]. Sustained control of serum blood glucose is the most important factor in mitigating the long-term health consequences of living with diabetes [Reference Stratton, Adler, Neil, Matthews, Manley and Cull6]. Thus, information about conditions that predispose individuals to be resistant to metabolic sensitizers is of clinical importance.
Lack of response to antidepressant medication is also an area of clinical concern [Reference Souery, Papakostas and Trivedi7], and it is known that early life adversity can impair response to treatments for depression [Reference Nemeroff, Heim, Thase, Klein, Rush and Schatzberg8]. It has also been suggested that metabolic impairment may contribute to treatment resistant depression, possibly via proinflammatory effects [Reference Pace, Hu and Miller9]. Insulin sensitizing medications have shown promise in improving response to standard treatments for depression [Reference Kemp, Ismail-Beigi, Ganocy, Conroy, Gao and Obral10–Reference Lin, Wroolie, Robakis and Rasgon13].
Pioglitazone is a PPAR-γ agonist in routine use in clinical care. It improves metabolic function via a diversity of mechanisms, including regulation of lipid metabolism, induction of mitochondrial uncoupling, inhibition of cortisol production, and downregulation of proinflammatory cytokines [Reference Berger and Moller14]. It has also been investigated for weight loss in obese, nondiabetic individuals, and found to reduce visceral fat [Reference Shea, Nicklas, Marsh, Houston, Miller and Isom15] and favor redistribution of fat to lower body depots, which are not associated with increased cardiovascular risk [Reference Shadid and Jensen16].
Our group has previously investigated the use of pioglitazone as an augmenting agent for depression in a randomized trial. Results from this trial [Reference Lin, Wroolie, Robakis and Rasgon13] indicated that pioglitazone treatment improved the response to usual care for depression among depressed, insulin-resistant individuals. This suggested that ability to respond to treatments for depression could be impaired by the presence of insulin resistance, and that this barrier could be overcome by treatment with pioglitazone.
Because both insulin resistance and chronic depression are known to be adult sequelae of childhood adversity [Reference Campbell, Farmer, Nguyen-Rodriguez, Walker and Egede1, Reference Chapman, Whitfield, Felitti, Dube, Edwards and Anda2], we wished to investigate whether a history of early life adversity would affect response to treatment with pioglitazone. Specifically, we hypothesized that a history of early life adversity could engender a medication-resistant metabolic phenotype, such that individuals with histories of childhood adversity would see more moderate responses to treatment with pioglitazone than those without such histories.
2. Aims and hypotheses
Our first aim was to determine whether a history of childhood adversity would affect response to pioglitazone. We predicted that a history of early life adversity (measured by score on the Childhood Trauma Questionnaire, CTQ) would interact with study group assignment (pioglitazone or placebo) in affecting the changes in metabolic parameters and depressive symptoms observed over the twelve weeks of the study.
Our second aim was to determine whether, among individuals treated with pioglitazone, those with histories of early life adversity would be more resistant to the beneficial effects of pioglitazone on both a) depressive symptoms and b) metabolic parameters. We predicted that higher CTQ scores would correlate with smaller observed reductions in depressive symptoms, glucose, insulin, and HOMA among individuals exposed to pioglitazone.
3. Methods
Methodology for this trial has been reported elsewhere [Reference Lin, Wroolie, Robakis and Rasgon13]. Briefly, nondiabetic individuals between 21 and 75 years old, having a body mass index (BMI) of 18.5–40 kg/m2, having at least 12 years of education, and having a history of depression with at least 8 weeks of stable TAU for depression, were recruited and randomized to receive twelve weeks of treatment with either placebo or 30 mg/day of pioglitazone. Forty-three subjects were recruited, of whom 38 completed the study: 21 in the pioglitazone group and 17 in the placebo group. Of the five dropouts, two were receiving placebo and three active drug. All subjects completed a CTQ. The CTQ is a 28-item self-report questionnaire that assesses exposure to five subtypes of early life adversity [Reference Bernstein, Stein, Newcomb, Walker, Pogge and Ahluvalia17]. Fasting plasma insulin and glucose as well as a two-hour oral glucose tolerance test (OGTT) were obtained at the beginning and end of the study. HOMA was calculated as (fasting glucose * fasting insulin)/405. Participants were categorized as insulin resistant if any of the following applied: baseline fasting glucose over 100, baseline fasting insulin over 15, or serum glucose of over 140 at 120 min post initial glucose challenge. Symptoms of depression were assessed with the Hamilton rating scale for depression (HAM-D) every two weeks for the duration of the study.
3.1. Statistics
For Aim 1, we used paired-sample T tests to confirm that pioglitazone was effective in improving metabolic parameters. We then used univariate ANCOVAs to look for associations between response to pioglitazone treatment (as measured by insulin, glucose, HOMA, OGTT, and HDRS-21) and early life adversity (CTQ total and each of five subscales).
For Aim 2, we plotted the associations between childhood adversity variables and changes in metabolic variables to observe their direction.
4. Results
4.1. Childhood adversity and pioglitazone treatment interact to effect changes in metabolism over time
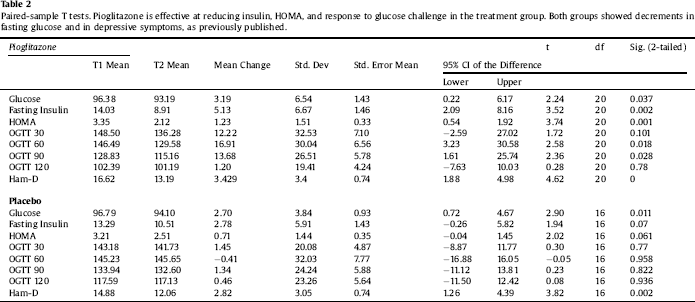
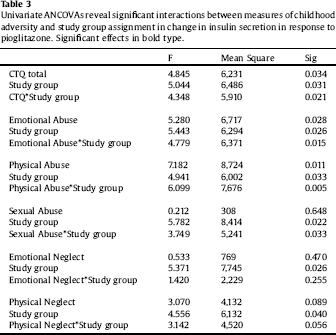
Clinical characteristics of the sample are displayed in Table 1. Paired-sample T tests confirmed the overall efficacy of pioglitazone for improving insulin sensitivity (Table 2). Univariate ANCOVA demonstrated a significant interaction between childhood adversity and study group assignment in determining changes in plasma insulin response to glucose challenge after 120 min, from beginning to end of the study (Table 3). Significant interactions were found between study group assignment and CTQ total, as well as separately for all CTQ subscales excepting physical neglect, in predicting changes in insulin secretion between the start and end of the study. No significant interactions were found for the outcomes of change in fasting glucose or glucose response to OGTT, or for changes in depression scores, although there was a trend for higher glucose response peaks in the OGTT among individuals with trauma histories. We performed a parallel analysis using a repeated-measures ANCOVA that covered all four time points in the glucose challenge test (0, 60 min, 90 min, and 120 min), and found substantially similar results (not shown).
Table 1 Characteristics of Study Subjects.
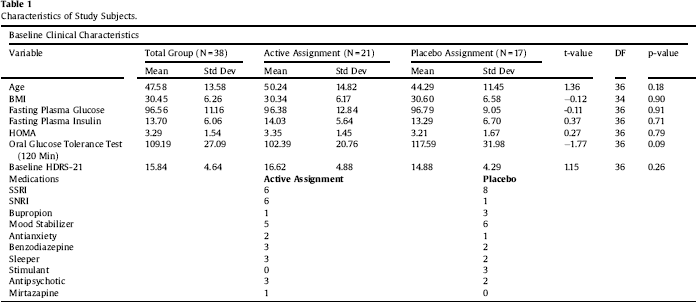
Table 2 Paired-sample T tests. Pioglitazone is effective at reducing insulin, HOMA, and response to glucose challenge in the treatment group. Both groups showed decrements in fasting glucose and in depressive symptoms, as previously published.
4.2 Direction of association between childhood adversity and changes in mood and metabolism
Higher rates of childhood adversity (physical abuse, physical neglect, emotional abuse, and total CTQ) were associated with increases in fasting plasma insulin after twelve weeks of treatment with pioglitazone (Fig. 1). Thus, the overall positive response to pioglitazone was driven by individuals without histories of childhood adversity. Additionally, the increased resistance to pioglitazone conferred by childhood adversity was not simply a function of greater baseline insulin resistance, as none of baseline fasting glucose, fasting insulin, or HOMA correlated significantly with the insulin response to pioglitazone treatment.
Table 3 Univariate ANCOVAs reveal significant interactions between measures of childhood adversity and study group assignment in change in insulin secretion in response to pioglitazone. Significant effects in bold type.
4.3. Insulin resistance at baseline
We also performed an additional analysis examining changes in depressive symptoms, only among those patients who had insulin resistance at baseline (N = 20, of which 13 received pioglitazone and 7 received placebo). This was based on our previous finding that pioglitazone improved depressive symptoms only among those with insulin resistance at baseline [Reference Lin, Wroolie, Robakis and Rasgon13]. While the interaction between study group assignment and CTQ score was not significant in this limited sample (F = 4.71, mean square = 16.09, p = 0.072), the placebo group showed a trend towards less improvement in depressive symptoms with higher CTQ scores, whereas the pioglitazone group showed no such trend (Fig. 2). No interaction was found between study group assignment and CTQ score with respect to changes in depressive symptoms among the insulin sensitive group (p = 0.931).
5. Conclusions and discussion
Our finding that childhood adversity impairs metabolic response to pioglitazone highlights the potential that early life adversity may have far-reaching negative effects on physiology over the lifespan by interfering with the body’s ability to respond to available treatments.
5.1. Multiple subtypes of childhood adversity contribute to metabolic resistance to pioglitazone
While only emotional abuse was associated with baseline metabolic factors, metabolic changes in response to pioglitazone were significantly affected by total CTQ as well as by the subscales of emotional abuse, physical abuse, and physical neglect. The use of a randomized intervention created a larger separation between the control and pioglitazone populations, likely amplifying our ability to detect the effect of interest. While it is quite likely that distinct subtypes of childhood adversity operate on metabolism via distinct mechanisms, it is also possible that the differences we found among CTQ subscales represent stochastic variation related to small sample size. Larger studies of this type would be needed to sort out these questions.
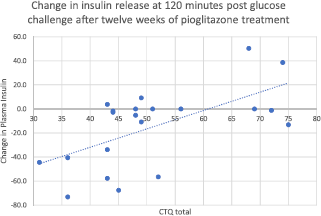
Fig. 1. Change in insulin release at 120 min after glucose challenge, after twelve weeks of treatment with pioglitazone. Total CTQ is significantly associated with nonresponse to pioglitazone by repeated-measures ANOVA on the pioglitazone group only, N = 21. (F = 11.539, mean square = 6714, p = 0.003).
While we examined changes in fasting glucose and oral glucose tolerance as well, insulin response to glucose load was the only outcome that was clearly affected by childhood adversity. It may be that fasting insulin represents a ‘cleaner’ outcome, one more heavily determined by internal biological factors and less susceptible to perturbation by recent, short-term environmental effects than serum glucose.
5.2. Effects of baseline insulin resistance on response to treatments for depression
This trial was originally designed to investigate whether pioglitazone could facilitate response to standard treatments for depression. The treatment and control groups overall did not separate on our depression measures: all individuals received treatment as usual for depression, and depressive symptoms fell significantly in both groups. In the initial study, a model that included baseline insulin resistance revealed that only insulin resistant subjects benefited from pioglitazone augmentation for depression [Reference Lin, Wroolie, Robakis and Rasgon13]. We therefore repeated our analysis in the current study while limiting our sample pool to only those participants with baseline insulin resistance. However, because we were examining an interaction with a covariate, our study power was more limited, and thus the further power reduction imposed by limiting the analysis to insulin resistant individuals brought any effects below the limit of detection.
Nonetheless, the observed trends indicated that among those insulin resistant participants who received placebo, higher CTQ scores were associated with continuing depression over the course of the study, whereas among insulin resistant participants who received pioglitazone, depression tended to improve regardless of CTQ score (Fig. 2). These trends are consistent with the hypothesis that childhood adversity confers a combined phenotype of depression and insulin resistance, which is partially reversed by insulin sensitizing medication, permitting depressed, previously insulin resistant individuals to respond more effectively to standard treatments for depression. It is possible that if we had a larger sample of insulin resistant individuals, this observed trend for an interaction between childhood adversity and study group assignment for the outcome of depressive symptoms could have reached significance.
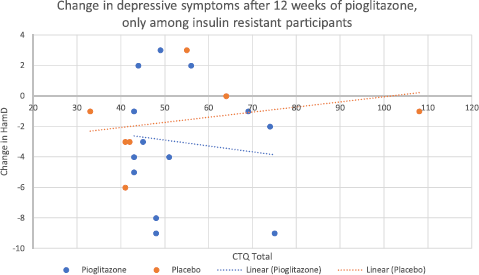
Fig. 2. Change in Hamilton Depression scores, after twelve weeks of treatment with either pioglitazone or placebo, only among those insulin resistant at baseline (N = 20). The interaction between CTQ score and study group assignment with respect to the outcome of depressive symptoms did not reach significance (p = 0.072). However, there was a trend for less improvement in mood with increasing CTQ score only in the placebo group, whereas no such trend was evident for the pioglitazone group.
Early life adversity has long been known to confer resistance to standard psychopharmacological treatments for depression [Reference Nemeroff, Heim, Thase, Klein, Rush and Schatzberg8]. Our work suggests that metabolic dysfunction could be a mediating factor in the relationship between childhood adversity and resistance to treatment for depression.
5.3. Allostatic load as a determinant of responsiveness to treatment
Our results suggest that early life adversity confers a lasting metabolic change that impairs responsiveness to pioglitazone, certainly from a metabolic perspective and perhaps also from a psychiatric perspective. These results represent an interesting parallel to a previously published analysis of this cohort, which revealed that telomere length, another indicator of allostatic load, was positively associated with the antidepressant response to pioglitazone [Reference Rasgon, Lin, Lin, Epel and Blackburn18]. Telomere length was not, however, associated with the antidiabetic response to pioglitazone in that analysis. This suggests that allostatic load may include a number of different components that interact with PPARγ agonist action through distinct mechanisms to affect mood and metabolism. The component of allostatic load reflected by telomere length may be most relevant to mood symptoms, while the component reflected by childhood adversity score may be more highly relevant to metabolic symptoms. As PPARγ is a transcription factor whose activation initiates multiple parallel downstream cascades, it is quite possible that the aspect of PPARγ action that interacts with telomere length to affect mood may be distinct from the aspect that interacts with childhood adversity score to affect metabolism.
5.4. Potential mechanisms
We speculate that increased levels of inflammation could have mediated the effects observed in this study. Insulin resistance, childhood trauma history, and depression are known to have overlapping and related inflammatory components. Type II diabetes and depression share a common molecular signature of increased inflammation, involving elevation of C-reactive protein (CRP), tumor necrosis factor alpha (TNFα), and interleukin-6 (IL6), and possibly depression of transforming growth factor beta (TGFβ) and adiponectin [Reference Furtado and Katzman19–Reference Liu, Guo, Zhang, Cheng, Liu and Ding22]. Childhood trauma is also known to be associated with proinflammatory phenotypes in adulthood, including elevation of CRP and TNFα, and possibly also IL-6 [Reference Baumeister, Akhtar, Ciufolini, Pariante and Mondelli23].
PPARγ is known to affect insulin sensitivity via pleiotropic mechanisms, including glucose homeostasis, lipid metabolism, and adipokine regulation [Reference Choi, Park and Choi24]. Adipokine mediators of PPARγ action include adiponectin, TNFα, and IL-6, all of which have been repeatedly associated with depressive symptoms. Additionally, animal work has shown that the antidepressant-like effects of pioglitazone are associated with PPARγ-mediated reduction in inflammatory cytokines, including expression of TNFα, and IL-6, in murine hippocampal microglia [Reference Zhao, Wu, Yan, Xie, Fan and Zhang26].
It is thus possible that individuals with histories of childhood trauma have constitutively higher levels of TNFα, IL-6, and perhaps other relevant inflammatory mediators, and that chronic high levels of inflammatory signaling result in lowered sensitivity to the perturbations in these pathways induced by PPARγ activation. Thus people without childhood trauma histories and with constitutively low levels of inflammatory mediators may display greater responsiveness to the alterations in these pathways produced by pioglitazone treatment, whereas individuals with trauma histories who express higher levels of the above inflammatory mediators may have downregulated the relevant receptors in these signaling pathways, and thus be less susceptible to pioglitazone-induced alterations in ligand levels.
Unfortunately we did not have any direct data on inflammatory markers in the current study cohort that would help to support or disprove the above hypothesis.
5.5. Limitations
The main limitation of this study was its small sample size. This is the likely explanation why we did not detect broader associations between early life adversity and adult metabolic impairment and depression, and also posed a limitation to our ability to stratify by baseline insulin resistance, which would likely have yielded further important insights. As this was among the earliest randomized controlled trials of metabolic sensitizing agents for depression, our results can be used to estimate effect sizes for future, more appropriately powered studies. The randomized interventional design was the study’s greatest strength, as individual differences in treatment response are very difficult to detect in observational investigations.
5.6. Future directions
Our findings have clinical importance in that they indicate that patients with metabolic dysfunction and histories of childhood adversity may be less likely to respond to antidiabetic medications. Intriguingly, they also suggest that among individuals with both insulin resistance and depression, insulin-sensitizing treatment may disrupt the association between childhood adversity and poor response to treatment for depression.
Larger and more effectively powered studies are needed to confirm these results. If confirmed, our results could indicate that assessment of childhood history may assist clinicians in identifying patients who may be at risk for treatment-resistant diabetes. Future investigations would then be needed to identify methods for overcoming such treatment resistance.
Author statement
Thalia Robakis conceived of the question, analyzed the data, and wrote the paper, with input and assistance from other authors. Kathleen Watson provided statistical oversight and conceptual input. Alison Myoraku assisted with data processing, construction of tables, and editing and formatting of references. Tonita Wroolie obtained the CTQ data from study participants. Carla Nasca, Benedetta Bigio, and Bruce McEwen contributed conceptual input and comments/editing on the manuscript. Natalie Rasgon conceived of, funded, and conducted the original parent study and maintains proprietorship of the data, and also contributed conceptual input to this analysis.
Conflict ofinterest
Dr. Rasgon has received research support from Corcept Pharmaceuticals, Bayer, Abbot, Forest Laboratories, GlaxoSmithKline, Pfizer, and Magceutics, and has received consultant or speaker fees from Takeda, Shire, Sunovion, Bristol-Myers Squibb, Pfizer, and Wyeth. Drs. Robakis, Watson, Wroolie, Myoraku, Nasca, Bigio, and McEwen have nothing to disclose.
Funding
Funding for this research was provided by the National Institutes of Mental Health grant 1R21 MH093948-01A1, to principal investigator Dr. Rasgon. Drs. Nasca, Bigio, and McEwen were also supported by the Hope for Depression Research Foundation.










Comments
No Comments have been published for this article.