1. Introduction
Gender dysphoria refers to a mismatch between the biological sex and gender assigned at birth and the individuals felt gender identity. The felt mismatch may result in a strong desire to be treated as the other gender or to transition to the other gender [Reference American Psychiatric Association1]. Studies suggest a prevalence rate for transsexualism of 4.6 in 100,000 individuals; 6.8 for transgender women and 2.6 for transgender men [Reference Arcelus, Bouman, Van Den Noortgate, Claes, Witcomb and Fernandez-Aranda2]. Autism spectrum disorder (ASD) is characterised by deficits in social communication and repetitive and stereotyped behaviours [Reference American Psychiatric Association1] with research suggesting a prevalence rate of 1 in 59 with occurrences between three and four times higher in boys [Reference Prevention. CfDCa3, Reference Loomes, Hull and Mandy4]. Both ASD and gender dysphoria are relatively rare conditions, and comorbidity rates would be expected to be low for this population; however, studies suggest that the full criteria for ASD, or the presence of elevated autistic traits, is higher in individuals identifying as transgender than in the general population [5–Reference Pasterski, Gilligan and Curtis7]. In gender dysphoric children, de Vries et al. [Reference de Vries, Noens, Cohen-Kettenis, van Berckelaer-Onnes and Doreleijers5] reported a rate of ASD approximately 37 times higher than would be expected in the general population. Research into this area has the potential to provide new therapeutic approaches to treating individuals with gender dysphoria who display a high level of ASD traits and to also confirm or challenge the current theoretical understandings of ASD.
ASD traits in transgender individuals may be higher in transgender men than in transgender women [Reference Jones, Wheelwright, Farrell, Martin, Green and Di Ceglie6]. The Extreme Male Brain theory [Reference Baron-Cohen and Hammer8] suggests that individuals with ASD display an extreme version of the male cognitive profile and its associated behaviours, and hypothesises that ASD individuals assigned female at birth (AFB) should display hyper-masculinised traits [Reference Baron-Cohen and Hammer8]. Scores on the Autism Quotient (a screening tool for ASD) are reported to be higher in transgender males than transgender females [Reference Jones, Wheelwright, Farrell, Martin, Green and Di Ceglie6], and transgender males score lower on measures of empathy than cisgender females [Reference Di Ceglie, Skagerberg, Baron-Cohen and Auyeung9]. It has been suggested that this hypermasculine state may lead women with ASD to feel separated from their traditional gender identity [Reference Cooper, Smith and Russell10] and may lead some women to develop gender dysphoria. Although the theory does not explicitly make predictions about sexuality, it might be expected that in ASD individuals assigned male at birth (AMB) hypermasculinity would lead to fewer cases of gender dysphoria. However, research has shown that within individuals with gender dysphoria, both those AMB and AFB show an overrepresentation of autism traits as measured on the Autism Quotient and Social Responsiveness scale [Reference Heylens, Aspeslagh, Dierickx, Baetens, Van Hoorde and De Cuypere11].
Research into gender fluidity and ASD has tended to focus on individuals who see their gender in terms of binary distinctions, although historically attempts have been made to categorise individuals with gender dysphoria along the lines of sexual preference and age of onset [Reference Lawrence12]. The Extreme Male Brain theory has been criticised for stereotyping behaviour as either exclusively male or female [Reference Jack13], and research into gender identity and ASD has often excluded nonbinary individuals. Research suggests that there are higher occurrences of homosexuality, bisexuality and asexuality in ASD samples [Reference George and Stokes14], gender-nonconformity is higher in individuals with ASD [Reference Rudolph, Lundin, Åhs, Dalman and Kosidou15] and especially so in women [Reference Dewinter, De Graaf and Begeer16]. At present, little is known about individuals who self-identify as nonbinary. Research conducted in the Netherlands [Reference Kuyper and Wijsen17] reported that 4.6% of individuals AMB and 3.2% of individuals AFB reported an ‘ambivalent gender identity’ (defined as equal identification with the other sex as with their sex assigned at birth); similar findings have been reported in a Belgian sample [Reference Van Caenegem, Wierckx, Elaut, Buysse, Dewaele and Van Nieuwerburgh18]. Concerning autistic traits, one of the few published papers in this area reported that individuals who identified as ‘genderqueer’ (a nonbinary identity), scored higher on measures of autistic traits than both cisgender and binary transgender participants [Reference Kristensen and Broome19].
While best practice for treating and assessing transgender individuals has included many areas of concern [Reference Wylie, Barrett, Besser, Bouman, Bridgman and Clayton20], there has been a neglect of possible autistic traits and their impact on treatment within this community [Reference Doward21, Reference Strang, Meagher, Kenworthy, de Vries, Menvielle and Leibowitz22]. Problems in interpreting social signals, a literal understanding of language and problems in recognising and interpreting one’s own emotions may mean that transgender individuals displaying high ASD traits struggle with therapeutic interventions [Reference Jacobs, Rachlin, Erickson-Schroth and Janssen23]. Therefore, research is needed to establish the core ASD traits that are apparent in both transgender and nonbinary individuals. This paper investigates autistic traits and cases of autism in individuals who do not identify as cisgender and further explores the differences between binary and nonbinary categories. To establish a profile of autism, the study used the Autism Quotient [Reference Baron-Cohen, Wheelwright, Skinner, Martin and Clubley24], the Empathy Quotient [Reference Baron-Cohen and Wheelwright25], the Systemising Quotient [Reference Wheelwright, Baron-Cohen, Goldenfeld, Delaney, Fine and Smith26] and the Reading the Mind in the Eyes task [Reference Baron-Cohen, Wheelwright, Hill, Raste and Plumb27]. Furthermore, we examined the impact of gender assigned at birth on these measures.
2. Method
2.1 Participants
Data were collected using Qualtrics from 196 participants over the age of 18 who self-identified as either cis-gender, binary transgender (male or female) or nonbinary, constituting three groups in all. In total, 19 participants failed to complete the survey, and their data were removed. These incomplete responses were across all six gender identities: cisgender male (n = 3), cisgender female (n = 3), binary transgender male (n = 6), binary transgender female (n = 2), nonbinary AMB (n = 1) and nonbinary AFB (n = 4). Within the remaining 177 participants, 66 were AMB and 111 were AFB; the three groups were matched on age (F(1,2) =.23, p =.98). The survey was distributed on eight Facebook groups, which acted as support groups for transgender individuals. Two of these groups were secret groups which are untraceable online and were provided to the study through members of the transgender community; the remainder were closed groups. The survey was also shared on three other general Facebook groups which targeted Anglia Ruskin students, where data from cisgender participants was collected. Of those participants identifying as transgender, 74% were receiving or had received support from a gender identity clinic, 22% had received no support, and 4% preferred not to say. In the nonbinary group, 42% were receiving or had received support from a gender identity clinic, 54% had not, and 4% preferred not to say.
2.2 Ethics
Ethical approval for the survey was received from the Psychology Departmental Research Ethics Panel (DREP) at Anglia Ruskin University (Approval code: EH16-025), in conformity with the British Psychological Society’s ethical guidelines and the World Medical Association Helsinki Declaration.
2.3 Materials
The Autism Spectrum Quotient [Reference Baron-Cohen, Wheelwright, Skinner, Martin and Clubley24] comprises 50 statements and is designed to screen for ASD. A cut-off of 32 was used to denote potential cases of ASD. A threshold score of 32 out of 50 has good discriminative validity for detecting adults with autistic traits (sensitivity = 76.71% and specificity = 74.07% [Reference Woodbury-Smith, Robinson, Wheelwright and Baron-Cohen28]. The test has good internal consistency (α = 79), and good test-retest reliability (r =.84; [Reference Stevenson and Hart29].
The Empathy Quotient [Reference Baron-Cohen and Wheelwright25] measures both affective and cognitive aspects of empathy across 40 items. Individuals with ASD score lower on this measure than typically developing individuals, with the majority of ASD individuals scoring less than 30 [Reference Baron-Cohen and Wheelwright25]. The EQ has been shown to have good test-retest reliability (r =.78–.97) and good internal consistency (α =.78–.92) [Reference Groen, Fuermaier, Den Heijer, Tucha and Althaus30].
The Systemising Quotient-Revised [Reference Wheelwright, Baron-Cohen, Goldenfeld, Delaney, Fine and Smith26] is a self-report measure consisting of 75 items, with a maximum score of 150. Individuals with ASD have a strong drive to systematise (analyse, control and utilise rule-based systems) and score higher on this measure than typically developing individuals. The SQ has good internal consistency (α =.79–.90) and good test-retest reliability (r =.79) [Reference Groen, Fuermaier, Den Heijer, Tucha and Althaus30].
The ‘Reading the Mind in the Eyes’ Test-Revised [Reference Baron-Cohen, Wheelwright, Hill, Raste and Plumb27] is a measure of advanced theory of mind, and individuals with ASD register scores lower than typically developing individuals. The RME test consists of 36 black and white photographs of facial expressions that are cut-off at the nose region, focusing on the eyes and forehead. Each expression is presented with four answer options. Reported internal consistency for the test ranges from α =.63 to 0.77 and the test has a test-retest reliability of r =.83 [Reference Vellante, Baron-Cohen, Melis, Marrone, Petretto and Masala31].
2.4 Procedure
Once distributed within the Facebook groups, any members could access the link to the survey from a computer. Informed consent was collected from all participants. The survey was completed in the following order: Autism Quotient, Empathising Quotient, Systemising Quotient-Revised and the Reading the Mind in the Eyes test. Once the last response was submitted from the RME, the participant was redirected to the debrief sheet, where they could either submit their results or withdraw by closing the browser.
2.5 Statistical analysis
Chi-square was used to test for associations between gender at birth and ASD status, and self-identified gender and ASD status and results are provided with odds ratios. To maximise statistical power, MANOVA was used for the first step in the analyses, with post hoc tests applied where appropriate. Bonferroni corrections were applied in the following manner. To control for Type 1 errors, when testing the multiple univariate interaction effects and the simple main effects for group, the alpha level was adjusted to 0.01. Bonferroni corrections were also applied to pairwise comparisons and p-values automatically adjusted in SPSS. Data met assumptions for MANOVA but violated the assumption of equal variances and covariances (<.001); since participant numbers in each cell were similar the MANOVA is robust to this violation [Reference Tabachnick and Fidell32], and the Pillai’s trace statistic was reported, which is robust to unequal covariance matrices [Reference Olsen33]. Groups were analysed in relation to their current gender identity and gender assigned at birth.
3. Results
3.1 Overview
Table 1 presents an overview of the data for each group. The question of whether the participant had a diagnosis of ASD was left blank by 18% of the respondents. A chi-square analysis demonstrated a significant association between the response to the question and group x 2(2) = 8.18, p =.02, φ =.22. The odds ratio of not responding in the cisgender group versus the transgender group is 4.23, 95% CI [1.46, 13.42] and in the cisgender group versus the nonbinary group 3.50, 95% CI [1.19, 10.69] and in the transgender versus nonbinary groups 0.80, 95% CI [0.30, 1.94]. There was also a statistically significant association between providing an answer to the question and gender assigned at birth x 2(1) = 12.25, p <.001, φ =.26. The odds ratio of not responding in the AMAB versus the AFAB group is 7.08, 95% CI [2.06, 24.36].
Table 1 Mean scores on each of the survey measures.

Autism Quotient (AQ), Empathising Quotient (EQ), Systematising Quotient (SQ), Reading the Mind in the Eyes Test (RME), Autism Quotient cut off (AQ CF). Absolute ranges: AQ (0–50), EQ (0–80), SQ (0–150), RME (0–36). Transgender male refers to individuals assigned female at birth abs transgender female refers to individuals assigned male at birth. In relation to the question asking participants whether they had a diagnosis of ASD 31 participants did not respond representing 18% of the data. These figures breakdown as: Cis- male 1(4%), cis female 4 (10%); transgender male 12 (39%), transgender female 1 (5%), nonbinary (AMAB) 1 (5%), nonbinary (AFAB) 12 (30%).
3.2 Rates of ASD
Within the combined transgender and nonbinary groups, 14% of participants reported having an ASD diagnosis, and in the cis-gender group, this figure was 4%. A chi-square test demonstrated a significant association between reported ASD diagnosis and self-defined gender, x 2(2) = 15.78, p <.001, φ =.33. The odds ratio of reporting an ASD diagnosis in the cisgender versus transgender group is 1.14, 95% [.18, 7.18] and in the cisgender versus nonbinary group 7.89, CI 95% [2.09, 29.65] and in the transgender versus nonbinary group 6.89, CI 95% [1.45, 32.90]. Of those reporting an ASD diagnosis, three participants from the cisgender group (all AMAB), one participant from the transgender group (AMAB) and one participant from the nonbinary group (AFAB) had AQ scores below 32. The analyses were run including and excluding these participants without a change in the results. The subsequent analyses reported include these five participants.
From those participants who did not report an ASD diagnosis, 28% of the combined transgender and nonbinary groups met the AQ cut-off score that would suggest the need for further screening for ASD, and none of the cisgender participants met this point. A chi-square test was conducted to investigate whether a relationship existed between AQ cut-off and self-defined gender, excluding the cisgender group. The chi-square result was not significant x 2(1) =.69, p =.41, φ =.08.
When gender at birth was examined, 10% of AFB participants reported having an ASD diagnosis, compared to 11% of AMB participants. A chi-square test of association demonstrated that there was no significant association between ASD diagnosis and gender at birth x 2(1) =.22, p =.88. For AFB participants not reporting an ASD diagnosis, 19% met the cut-off for ASD; in AMB participants, this figure was 9%. A chi-square test demonstrated that there was no significant association between ASD cut-off and gender assigned at birth x 2(1) = 1.82, p =.18.
3.3 Group comparisons on autism traits
A two-way MANOVA was run with two independent variables (gender at birth and self-identified gender) and four dependent variables (AQ, EQ, SQ and RME); the combined dependent variables were used to assess the levels of autistic traits.
The interaction effect between gender assigned at birth and self-identified gender on the combined variables was significant F(8338) = 2.57, p =.01, Pillai’s Trace =.12, η2 =.06. Examining the individual measures revealed a statistically significant interaction effect between self-identified gender and gender at birth for EQ F(2171) = 4.21, p =.01, η2 =.05 and SQ F(2171) = 6.80, p =.001, η2 =.07. No significant interaction effects were observed for either AQ F(2171) = 2.87, p =.06, η2 =.03 or RMA F(2171) = 2.28, p =.11, η2 =.03.
To understand the nature of the interactions, these effects were explored further. In relation to gender assigned at birth, there was a statistically significant difference between males and females on EQ scores in the cisgender group, with females scoring higher than males F(1171) = 4.79, p =.03, η 2 =.03, and on SQ scores with males scoring higher than females F(1171) = 9.53, p =.002, η 2 =.05. Gender differences on EQ scores for the transgender F(1171) = 3.03, p =.08, η 2 =.02 and nonbinary groups F(1171) =.59, p =.44, η 2 =.003 were not significant, nor were there any statistically significant differences in SQ for the transgender group F(1171) = 2.92, p =.09, η 2 =.02 or nonbinary group F(1171) = 1.31, p =.25, η 2 =.008.
In relation to self-defined gender, the post hoc analyses suggest that these results were driven by gender assigned at birth. There was a statistically significant difference between the three groups for those AFAB for EQ F(2171) = 16.49, p <.001, η 2 =.16, and SQ F(2171) = 19.21, p <.001, η 2 =.18, but not for those AMAB for either EQ F(2171) =.41, p =.66, η 2 =.005 or SQ F(2171) =.34, p =.72, η 2 =.004.
Follow up tests demonstrated that EQ scores for those AFAB were 17.12 (95% CI: 11–23.25) higher in the cisgender group than the transgender group (p <.001), and 12.95 (95% CI: 7–18.89) higher than the nonbinary group (p <.001). SQ scores for those AFAB were 24.02 (95% CI: -32.72 to -14.31) lower in the cisgender group than the transgender group (p <.001), and 25.65 (95% CI: -35.12 to -16.18) lower than the nonbinary group.
Finally, the main effect of group was significant F(8338) = 5.40, p <.001, η 2 =.11. The groups differed significantly on AQ, EQ and SQ, but not on RME. Post hoc analyses showed that these differences were between the cisgender and transgender group and the cisgender and nonbinary group on each of the dependent variables. There were no significant differences between the transgender and nonbinary groups on any of the dependent variables. The full results are presented in Table 2 (Fig. 1 and 2).
Table 2 Group main effect comparisons on the AQ, SQ, EQ, and RME measures.

** p <.01.
*** p <.001.
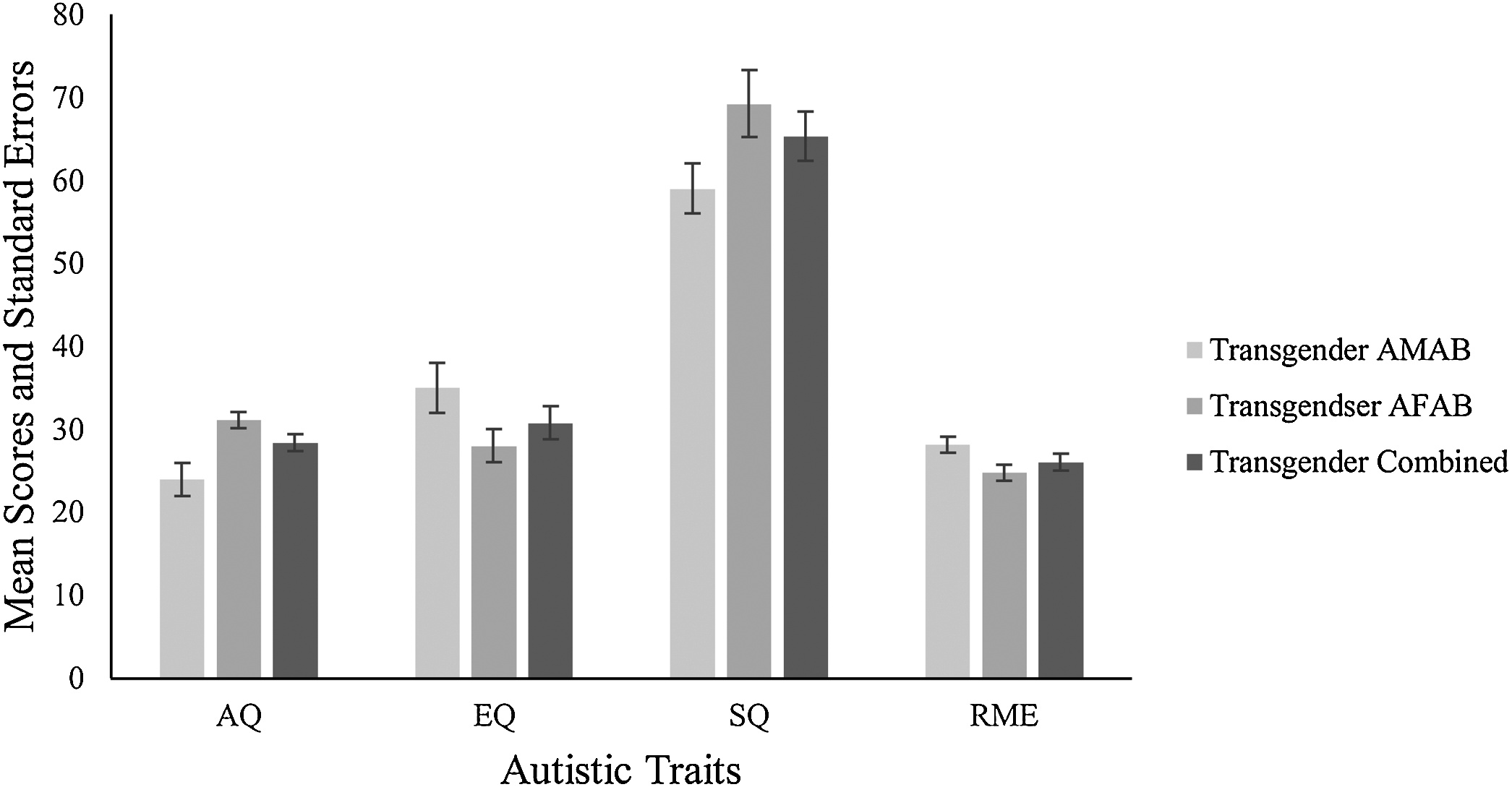
Fig. 1. Levels of autistic traits in individuals identifying as transgender.

Fig. 2. Levels of autistic traits in individuals identifying as nonbinary.
4. Discussion
4.1 Main findings
Both transgender and nonbinary groups participating in this study scored significantly higher on measures associated with ASD traits than a cisgender comparison group. Both groups also contained a higher than expected number of individuals who either had a diagnosis of ASD or met the AQ cut-off for ASD; AFB participants primarily drove these differences.
4.2 Meaning of the results
The findings concur with the extant literature that suggests a higher number of autism traits in individuals who identify as transgender. The rate of potential ASD in our study was higher than the prevalence of 0.6–1% of ASD in the general population [Reference Baird, Simonoff, Pickles, Chandler, Loucas and Meldrum34]. Of note is the fact that the proportion of individuals who either already had an ASD diagnosis or met the cut-off for ASD was higher in the nonbinary group than the transgender group; similarly, this group was also less likely to have visited a gender identity clinic.
Given the gender repartition in ASD is thought to be one female to every three males [Reference Loomes, Hull and Mandy4] finding an equivalent number of self-reported cases of ASD in AFAB and AMAB participants suggests that ASD is overrepresented in transgender and nonbinary AFAB individuals contrary to the conclusions of previous research [Reference de Vries, Noens, Cohen-Kettenis, van Berckelaer-Onnes and Doreleijers5]. Similarly, there was no association between reaching the AQ cut off and gender assigned at birth. However, these figures come from a self-selecting sample and need to be treated with caution. These result suggests the existence of a population of women reaching adulthood without their autism being formally detected and concords with current thinking that a significant proportion of women with ASD go undiagnosed [Reference Lai and Baron-Cohen35]. Although the AQ does not confer a diagnosis of ASD, our findings suggest ASD screening would be beneficial in specialist gender clinics, and the formal identification of ASD could potentially lead to a more appropriate level of support.
The gender analyses reported in our study potentially supports the Extreme Male Brain theory of ASD. However, it is noted that transgender and nonbinary AMB individuals also had elevated AQ scores, with 11% of this group reaching the cut-off for ASD; this suggests a more nuanced theory is needed.
While our findings suggest that there may be a high number of undiagnosed individuals with ASD in transgender and nonbinary populations, an alternative interpretation is plausible. Raised scores on the AQ and SQ, and lower scores on the EQ, rather than being unique to ASD, may be a feature of any condition where the developing individual has experienced feedback counter to their subjective experience of the self; we could call these out of kilter identities. In the case of transgender and nonbinary individuals, a disconfirmation of the self during development may result in the application of a systematic rather than empathetic style of cognition. Another possible explanation for the findings is that transgender individuals show specific but limited autistic traits confined to the social domain [Reference Nobili, Glazebrook, Bouman, Glidden, Baron-Cohen and Allison36] due to societal rejection; however, research suggests that children and adolescents with gender dysphoria show elevated levels of all autistic traits [Reference van der Miesen, de Vries, Steensma and Hartman37].
4.3 Clinical implications
Our research suggests that professionals treating transgender and nonbinary individuals need to consider the potential presence of autistic traits, particularly in AFB individuals. This is important as research demonstrates that being a minority within a minority group increases susceptibility to mental health conditions [Reference George and Stokes38]. Research suggests that consensus amongst clinicians working in this area can be achieved and guidelines established [Reference Strang, Meagher, Kenworthy, de Vries, Menvielle and Leibowitz22]. Focus on ASD traits is pertinent, given that the UK Gender Identity Development Service reports that AFB individuals are nearly two times more likely to be referred to specialist gender clinics than AMB individuals [39]. Our study confirmed reports of lower EQ scores in transgender and nonbinary AFB participants compared to cisgender females [Reference Di Ceglie, Skagerberg, Baron-Cohen and Auyeung9], and this group also demonstrated higher SQ scores when compared to cisgender females. This rigid cognitive profile relies on rule-based processing rather than the ability to intuit meaning from the non-verbal communication of others and so may create challenges in therapy and counselling [Reference Jacobs, Rachlin, Erickson-Schroth and Janssen23]. Rigidity of thought may also render the acceptance of gender variant feelings difficult [Reference Hannah and Stagg40]. Clinicians and healthcare providers need to be prudent with their choice of words; implied meanings, metaphors or idiomatic language might not be understood in the manner in which it is intended [Reference Chahboun, Vulchanov, Saldaña, Eshuis and Vulchanova41]. High scores on the SQ suggest that the transgender and nonbinary AFB groups would benefit from precise causal explanations, and they may seek simple or overly logical explanations of complex matters, thus resulting in frustration when unequivocal answers are not possible. Understanding of this cognitive style may reduce frustration, miscommunication and withdrawal from support.
4.4 Limitations
As with all internet surveys, self-selection and participants’ understanding of the survey requirements are difficult to gauge. Participants self-defined their gender, and while the study benefited from reaching participants that may never present at a gender identity clinic, we cannot be sure that all participants fully understood the gender categories. For example, the nonbinary identity is an emerging term in gender research, and it is possible that some participants may have failed to identify binary and nonbinary identities as separate categories. We also acknowledge that gender identity is not stable and that the individuals taking part in our study are likely to define their identity differently at varying stages in their lives; for example, gender may be defined differently when socially transitioning (e.g. coming out) and/or medically transitioning (e.g. using hormones/surgery).
Contributions
SS contributed to the design of the study, data analysis and the final write up of the study and final approval of the manuscript.
JV contributed to the design of the study, data collection and data analysis and final approval of the manuscript.








Comments
No Comments have been published for this article.