Dural arteriovenous fistulas (DAVFs) are rare intracranial arteriovenous malformations (AVM) characterized by direct shunting between meningeal arteries and dural sinuses, dural veins, or cortical veins. Multiple classification systems have been devised for DAVFs, including the Borden et al.Reference Borden, Wu and Shucart1 and Cognard et al.Reference Cognard, Gobin and Pierot2 classifications. Both of these classifications are predominantly based on the pathway of venous drainage and presence of antegrade or retrograde venous flow, with higher grade lesions (Borden II–III and Cognard IIa–V) portending a poorer prognosis. DAVFs with retrograde leptomeningeal venous drainage (RLVD) are more likely to present with severe symptoms.Reference Houser, Hillier, Baker, Albert, Rhoton and Okazaki3
DAVF with multiple arterial feeders, or small tortuous arterial supply, can be difficult to treat with trans-arterial embolization (TAE). In these cases, transvenous access to the fistula offers an alternate route for treatment. Transvenous embolization (TVE) approaches have been well established as a safe and effective method for treatment of DAVFs involving dural sinuses including cavernous sinus, transverse sinus, sigmoid sinus, and lesions at the cranio-cervical junction.Reference Roy and Raymond4 For DAVF draining directly into the cortical veins (Borden type 3), TAE techniques remain the preferred initial approach for treatment.Reference Choo and Shankar5 We present two cases of Borden type 3 DAVF treated with TVE through the draining leptomeningeal veins.
In case 1, a 72-year-old presented with a left cerebellar hemisphere acute intraparenchymal hemorrhage. A diagnostic cerebral angiogram was performed which demonstrated a Borden type 3 DAVF along the left tentorial leaflet near the petroclival junction (Figure 1). Arterial supply to the lesion was from enlarged tentorial and meningeal branches of the left internal carotid artery (ICA) and petrous branches of the left middle meningeal artery (MMA). Venous drainage of the DAVF was through the left basal vein of Rosenthal, into the vein of Galen with evidence of RLVD into multiple cerebellar veins (Figure 1).
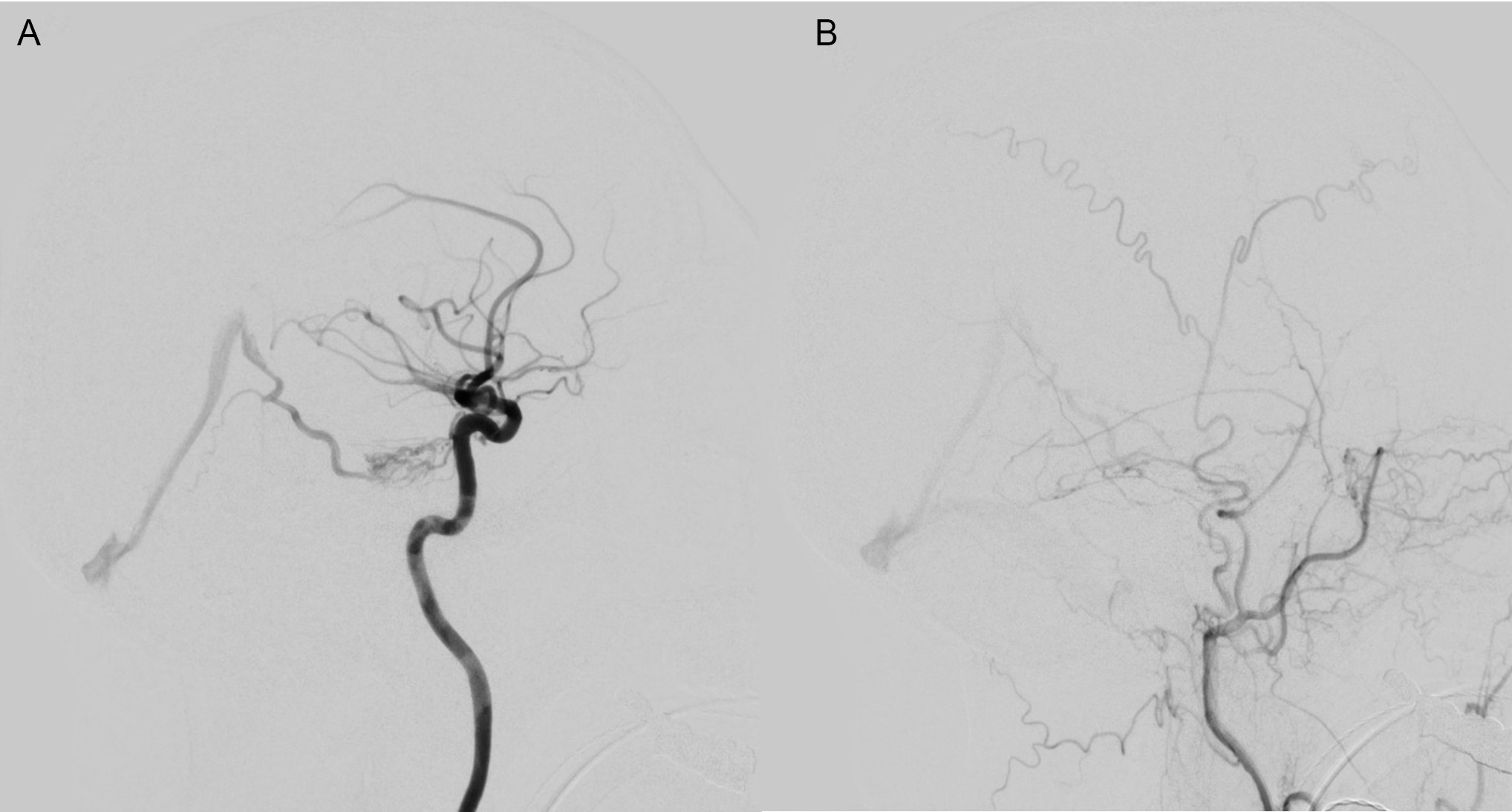
Figure 1: Case 1 Pre-embolization angiography images showing meningeal branches of the left ICA (A) and petrous branches of the left middle meningeal artery (B) supplying a type 3 DAVF with cortical venous drainage primarily into the vein of Galen.
The patient underwent surgery for attempted resection of the lesion, but a subsequent cerebral angiogram showed residual. The residual DAVF was supplied by tiny tentorial and meningeal branches arising from the left ICA and left MMA (Figure 1). Based on the angioarchitecture of the lesion, an endovascular treatment via a transvenous route was pursued. Under general anesthesia, a 6-French femoral sheath was placed in the right femoral vein, and a 5-French femoral sheath was placed in the left femoral artery for control angiogram. Under roadmap guidance, a triaxial system with a Sofia 125-centimeter (cm) intermediate catheter, Scepter XC balloon (MicroVention Inc., Tustin, CA, USA) microcatheter, and a Transend-14 microguidewire (Stryker Neurovascular, Fremont, CA, USA) for guidance was advanced into the left basal vein of Rosenthal through the sigmoid sinus and straight sinus. The microcatheter was advanced into the fistula and the balloon was partially inflated (Figure 2). Ethylene vinyl alcohol copolymer (Onyx-18; eV3 Endovascular, Inc., Plymouth, Minnesota) was then prepared according to the directions for use and the dead space within the microcatheter was filled with dimethyl sulfoxide. Under roadmap guidance, Onyx-18 was slowly injected into the site of fistula with gradual filling of the draining vein and retrograde filling of the distal portion of the feeding arteries was identified. Onyx-18 injection was terminated at this point. Left-sided internal and external carotid artery control angiograms showed no residual filling of the DAVF (Figure 2).
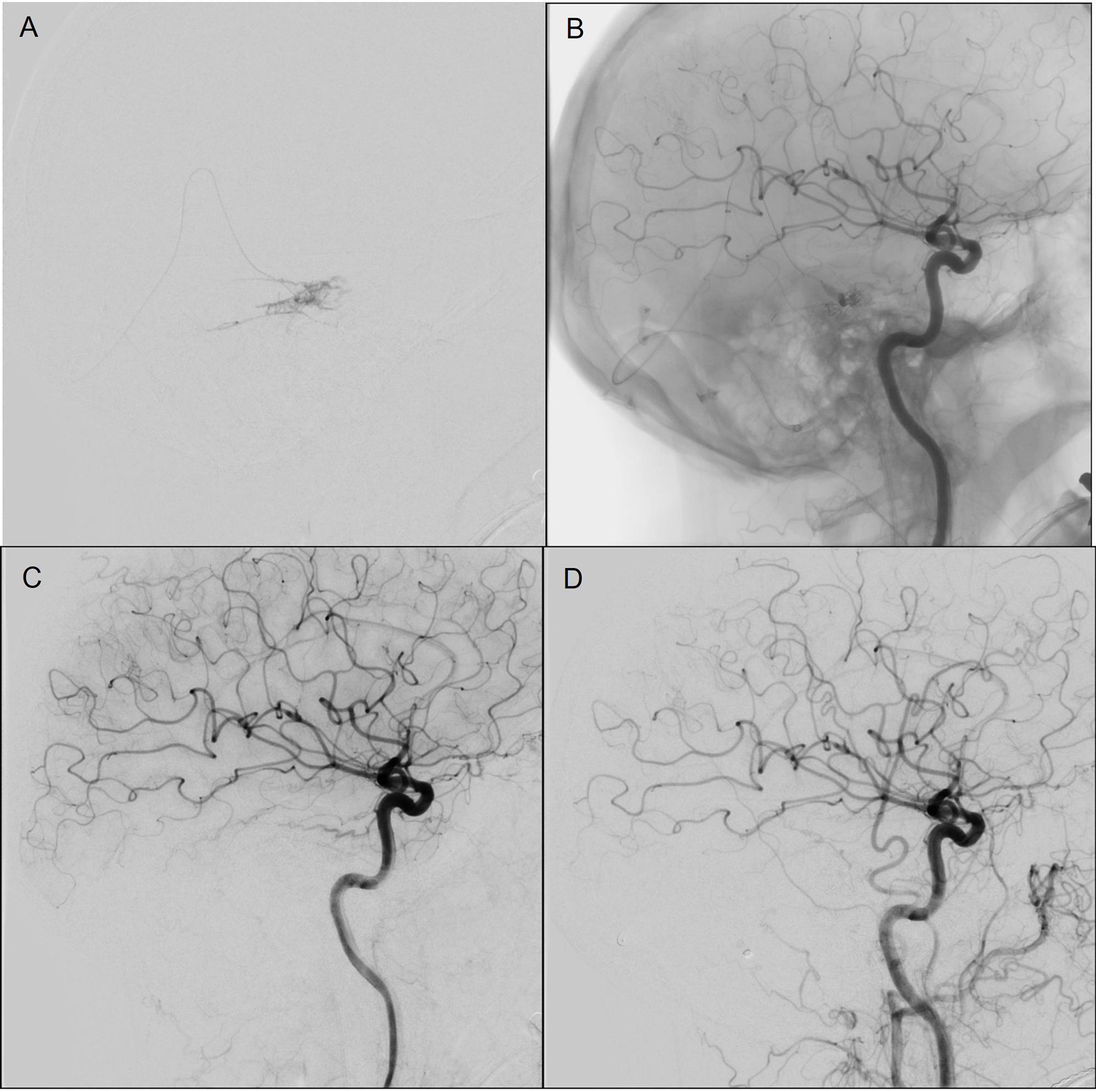
Figure 2: Case 1 (A and B) Angiography images during transvenous Onyx injection with balloon in situ to protect from reflux. Angiography images immediately following transvenous embolization Left ICA (C) and Left common carotid artery (D).
Follow-up time of flight and contrast-enhanced magnetic resonance angiogram at 5 months and 1 year following embolization demonstrated no evidence of residual or recurrent filling of the DAVF. The follow-up diagnostic cerebral angiogram was declined by the patient.
Case 2 pertains to a 47-year-old female with a history of breast cancer presenting to the emergency department with sudden onset diplopia, tinnitus, and headache. An initial noncontrast CT showed a small intraparenchymal hemorrhage in the right paramedian dorsal midbrain. A follow-up MRI with time-of-flight angiography of the circle of Willis showed multiple small vessels coursing toward the vein of Galen, as well as asymmetrical enlargement of the right internal cerebral vein, which raised suspicion for an underlying vascular malformation. This was confirmed on conventional cerebral angiography to be a Borden type 3 DAVF (Figure 3). The DAVF was located along the tentorium and was supplied by hypertrophied meningeal branches from the left MMA, right ICA, and posterior meningeal branches arising from the right vertebral artery. The DAVF was drained by an enlarged leptomeningeal vein draining into the vein of Galen. Venous reflux toward the left transverse sinus was also noted (Figure 3). A decision to pursue endovascular management was made.
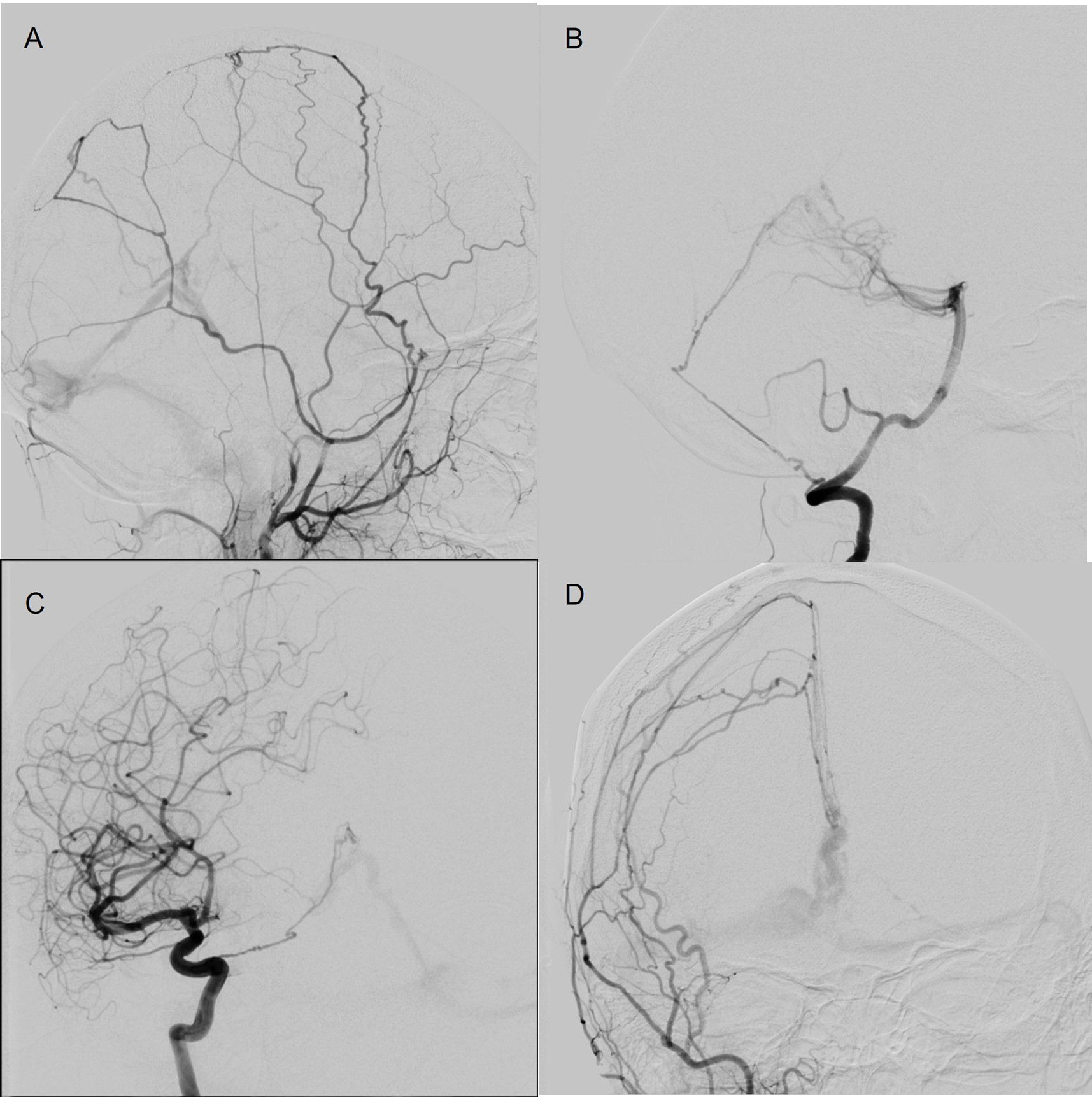
Figure 3: Case 2 Diagnostic cerebral angiogram showing multiple meningeal arteries supplying the type 3 DAVF arising from the left MMA (A), right vertebral artery (B), right ICA (C), and right external carotid artery (D).
Under general anesthesia, a 6-French sheath was introduced into the right femoral artery, and diagnostic angiograms in the left common and external carotid arteries were performed (Figure 3). Under roadmap guidance, a SONIC microcatheter (Balt, Montmorency, France) with Hybrid 0.007 microguidewire was navigated into the left MMA but could not be advanced very close to the site of fistula (Figure 4A). An attempt to embolize at this level with injection of 25% n-butyl-2-cyanoacrylate glue and lipiodol was made. The glue reached the site of the fistula, but there was persistent filling of the fistula. The decision was then made to pursue TVE of the DAVF. A 6-French sheath was placed in the left common femoral vein, and a 6-French guiding catheter was introduced into the right sigmoid sinus. Using a Headway Duo microcatheter (MicroVention Inc., Tustin, CA, USA) and a Transend-14 microguidewire (Stryker Neurovascular, Fremont, CA, USA), the draining leptomeningeal vein was accessed, and the catheter was advanced to the site of fistula (Figure 4B). Once at the fistula, five Optima 10.4 mm coils (Balt, Montmorency, France) between 8 and 13 cm in length were deployed that resulted in decreased flow through the fistula (Figure 4C). Further embolization with injection of 0.8 milliliters (mL) of Onyx-18 was then performed and the microcatheter was withdrawn. A control right common carotid artery run demonstrated complete occlusion of the DAVF.
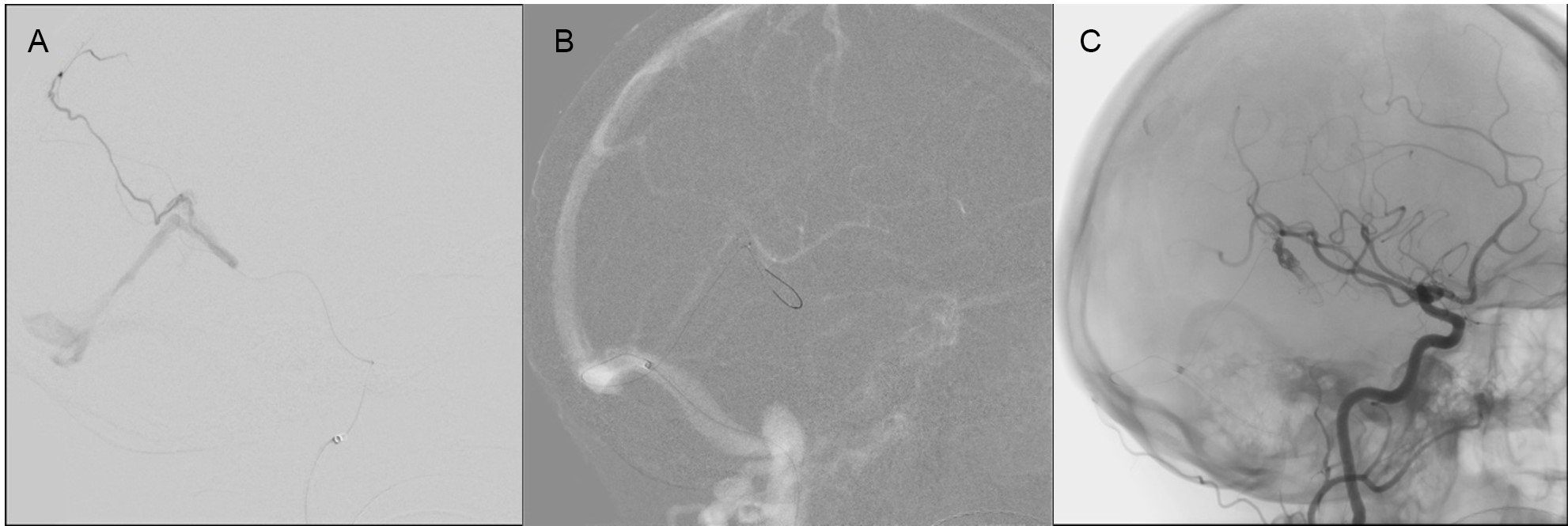
Figure 4: Case 2 Left MMA cannulation with an inability to advance close to the fistula (A). Selected roadmap images for microcatheter advancement to the site of fistula through draining cortical vein (B). Immediately following onyx injection (C).
One-year angiography follow-up revealed no residual arteriovenous shunting through the treated DAVF.
DAVFs are rare and complex arteriovenous shunts that are increasingly being treated through endovascular techniques. A multidisciplinary decision-making process involving both neurosurgeons and neurointerventionalists is mandatory. It is important to decipher the complex anatomy of these lesions for planning treatment. Digital subtraction angiography including 3D angiography helps to understand the anatomy, which assists in planning the endovascular treatment.Reference Botsford and Shankar6,Reference Botsford and Shankar7 Both TAE and TVE approaches have been described, but the majority of higher-grade lesions have been treated via a TAE. In cases where DAVF are fed by small, highly tortuous, or multiple bilateral arteries, the TAE can be challenging, and alternate approaches may be required.Reference Roy and Raymond4 We have presented two cases of successful TVE for treatment of Borden type III DAVF. In both cases, the TAE was initially attempted, but access to the fistulous point was challenging due to the arterial feeders being small, very tortuous and due to presence of dangerous arterial anastomosis. In such cases of high-grade DAVF, TVE is a valuable approach for successful treatment.
A recent meta-analysis by Fang et al.Reference Fang, Byun, Liu, Kings, Pereira and Brinjkji8 has confirmed that TVE through cortical veins is safe and effective for the treatment of brain AVM. Like the TVE of cerebral AVM, we used a combination of liquid embolic agent and coils in one case and liquid embolic agent through a balloon microcatheter in the second one. This approach has been increasingly recognized in cerebral AVM treatment for both its efficacy and safety.Reference Fang, Byun, Liu, Kings, Pereira and Brinjkji8 This approach is rarely needed for DAVF embolization with increasing experience with TAE of DAVF. Our two cases highlight potential use of TVE for Borden type 3 DAVF where TAE either fails or is difficult.
Although we did not experience any complications, TVE is not without risk with reported complication rates of 4%–9%.Reference Roy and Raymond4 Intracranial hemorrhage related to venous perforation due to mechanical perforation or injection-related increased intravenous pressure has been reported; however, the majority of these were reported before the implementation of many of the newer devices, and authors have suggested venous perforations can be avoided using test injections and slow injections. Furthermore, pial veins, which are more fragile, can hemorrhage causing significate morbidity. Transient or permanent neurological deficits related to venous congestion and venous drainage rerouting is another potential complication of TVE approaches which have been reported.Reference Kim, Kim and Sun9 Identification of the relevant angio-architecture and venous drainage pattern is critical to avoid venous congestion and rerouting. Despite this, TVE remains a safe and effective method for treatment of DAVF.
A major limitation is that we only presented two cases. However, we feel that TVE is a reasonable alternate approach to treat Borden type 3 DAVF, when TAE fails or is technically challenging. We recognize that case 1 had no angiographic follow-up to confirm complete occlusion as the patient refused. Despite this, we feel that the clinical and imaging stability on follow-up MRI studies at 5 months and 1-year post-procedure are sufficient to document successful treatment.
In these two cases, TVE was found to be feasible for treating Borden type 3 DAVF. This can be considered where access to the fistulous point is challenging due to complex arterial feeder anatomy or presence of dangerous arterial anastomosis.
Acknowledgments
None.
Funding
This study was not funded by any agency.
Conflict of Interest
The authors declare that they have no conflict of Interest.
Statement of Authorship
MN wrote the first draft of the manuscript and performed the chart reviews for the two cases. MN, NN, ZK, JS, and JS were involved in the design, literature search, and revision of the manuscript content. JS was involved in the conception, design, literature search, and revision of the manuscript content conception and finalized the manuscript. All author read and approved the final manuscript.
Copyright Statement
The corresponding author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license of permit for this article to be published (if accepted).








