Introduction
Neuromyelitis optica spectrum disorder (NMOSD) and myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease represent emerging autoimmune, demyelinating central nervous system (CNS) disorders. In recent years, it has become evident that NMOSD and MOG antibody-associated disease (MOGAD) are distinct from multiple sclerosis (MS), both clinically and with respect to treatment regimens and response. The identification of characteristic serum autoantibody biomarkers for NMOSD (anti-aquaporin-4) and MOGAD (anti-MOG), along with other paraclinical features such as neuroimaging and cerebrospinal fluid (CSF) analysis, has been game-changer for neurologists in distinguishing these disorders to optimize patient treatment.
The most recent diagnostic criteria for NMOSD emphasize the importance of anti-aquaporin-4 (AQP4) autoantibody status in confirming a diagnosis when coupled with an appropriate clinical presentation. Reference Wingerchuk, Banwell and Bennett1 These criteria have facilitated earlier diagnosis of anti-AQP4 seropositive NMOSD after the first episode of clinical disease. An earlier diagnosis allows for rapid initiation of relapse-preventing treatment; a crucial paradigm shift for patients given that disability in NMOSD, unlike MS, is almost entirely related to disease relapses. Reference Flanagan2
More recently, there has been increasing recognition that MOGAD is distinct from both anti-AQP4 seropositive NMOSD and MS. Reference Weber, Derfuss and Metz3 The pathogenicity of anti-MOG autoantibodies has not been confirmed, but the localization of MOG on oligodendrocytes and advances in laboratory detection of anti-MOG autoantibodies in characteristic CNS demyelinating syndromes (including upwards of 30% of “seronegative” NMOSD) have solidified the importance of MOGAD in a rapidly evolving field. Reference Zamvil and Slavin4–Reference Marignier, Hacohen and Cobo-Calvo6 While demographic, clinical, and radiologic differences between NMOSD and MOGAD are increasingly recognized, Reference Weber, Derfuss and Metz3,Reference Cobo-Calvo, Ruiz and Maillart7–Reference Lopez-Chiriboga, Sechi and Buciuc9 the diagnosis of MOGAD requires detection of serum anti-MOG autoantibodies. By contrast, seronegative NMOSD can be diagnosed without the presence of anti-AQP4 autoantibodies using 2015 diagnostic criteria; however, these criteria are more stringent. Reference Wingerchuk, Banwell and Bennett1 CSF anti-AQP4/MOG autoantibodies are not typically used for diagnosis, and their clinical significance remains uncertain given the peripheral immune origin of these antibodies. Reference Akaishi, Takahashi and Misu10,Reference Kwon, Kim and Kim11
Autoantibody status is also important for selecting the correct acute and preventative treatment strategies and may limit access to certain treatments. The use of recently proven highly effective targeted biologic therapies (eculizumab, inebilizumab, and satralizumab) in NMOSD is largely restricted to anti-AQP4 seropositive patients. Reference Pittock, Berthele and Fujihara12–Reference Cree, Bennett and Kim15 Although there are international recommendations for the diagnosis of MOGAD, Reference Jarius, Paul and Aktas16 formal consensus guidelines such as those for NMOSD Reference Wingerchuk, Banwell and Bennett1 have yet to be published. These MOGAD guidelines will presumably clarify distinctions between MOGAD and NMOSD given similar, albeit distinct clinical presentations and treatment responses. For example, patients with MOGAD appear to have greater relapse breakthrough while maintained on the B-cell depleting agent rituximab than do patients with anti-AQP4 seropositive NMOSD. Reference Durozard, Rico and Boutiere17 Furthermore, many disease-modifying therapies used in MS can exacerbate both NMOSD and MOGAD. Reference Flanagan2 These factors emphasize the need for accurate and reliable assays for anti-AQP4 and anti-MOG autoantibodies.
Cell-based assays (CBAs) have become an accepted standard for detection of autoantibodies against AQP4 and MOG Reference Waters, Pittock and Bennett18,Reference Waters, Komorowski and Woodhall19 . Broadly speaking, these CBAs utilize overexpression of either AQP4 or full-length human MOG on the surface of cognate cDNA-transfected cells in an indirect immunofluorescence assay utilizing a fluorochrome-conjugated anti-human IgG secondary antibody. Binding of the human antibody as reflected by fluorescence of the secondary antibody is either detected qualitatively by immunofluorescence microscopy or semi-quantitatively using automated flow cytometry (fluorescence-activated cell sorting; FACS). Reference Waters, Pittock and Bennett18,Reference Waters, Komorowski and Woodhall19 IgG anti-AQP4 CBAs have a reported sensitivity of 76%–94% and specificity of up to 100%. Reference Waters, Pittock and Bennett18,Reference Prain, Woodhall and Vincent20 IgG1 anti-MOG CBAs have a similarly high specificity of 98%–100% and a positive predictive value that is, based on recent studies, titre-dependent (72% for all titres, 82% for 1:100, 100% for 1:1000). Reference Waters, Komorowski and Woodhall19,Reference Sechi, Buciuc and Pittock21,Reference Manzano, Salky and Mateen22 CBAs are highly concordant comparing reputable laboratories. Notably, the discordance rate increases for IgG anti-MOG CBAs with borderline, low, or weak positive results, whereas IgG anti-AQP4 assay disagreement does not. Reference Reindl, Schanda and Woodhall23 Enzyme-linked immunosorbent assays (ELISA), immunoprecipitation, and indirect tissue immunofluorescence-based assays have fallen out of favour due to inferior sensitivity or specificity, although they may be the only assays available in certain countries. Reference Waters, Pittock and Bennett18,Reference Waters, Komorowski and Woodhall19
At our institution in Calgary, neurological services see patients from Southern Alberta, Eastern British Columbia, and Western Saskatchewan, with rare patients from Northern Alberta and the Northwest Territories. Although our centre has no standardized protocol for testing, patients seen in Calgary with clinical presentations concerning for NMOSD or MOGAD almost invariably undergo serum testing for anti-AQP4 and anti-MOG autoantibodies through the Calgary-based MitogenDx laboratory. When results deviate from expected, another serum sample may be outsourced to Mayo Clinic Laboratories (Rochester, MN, USA) at the discretion of the treating physician. This is often treated as a “confirmatory” test, despite the lack of a real-world comparison of the two laboratories. Both laboratories employ a CBA (see Materials & Methods). The cost of sending a patient sample for both anti-AQP4 and anti-MOG autoantibodies to Mayo Clinic is roughly six times the cost of MitogenDx testing. The frequency, rationale, and clinical utility of outsourced testing from our centre was poorly understood. Given the additional cost and lack of standardization in local practice, we undertook a quality improvement project to determine the added clinical value of this practice for patient care and a publicly funded healthcare system. Formal assay validation and accuracy assessments were not within the scope of this project.
Materials & Methods
Quality improvement approvals and study design
The requirement for Conjoint Health Research Ethics Board (CHREB: University of Calgary) approval was waived as this project met criteria for approval under the auspices of Quality Improvement (QI) by local pathways using the “A pRoject Ethics Community Consensus Initiative (ARECCI)” scoring tool through Alberta Innovates (https://arecci.albertainnovates.ca/resources/), a tool developed by the Alberta Research Ethics Community Consensus Initiative (ARECCI) Network (Supplemental Data, Figure S1). Approval was obtained from the Senior Consultant, Ethics Support, Health Systems Evaluation & Evidence, Provincial Clinical Excellence, Alberta Health Services (AHS), the AHS Privacy Advisor, and the Chair of Laboratory Medicine, Alberta, Canada, to proceed with this QI project. A retrospective review of all anti-AQP4 and anti-MOG autoantibody tests performed at MitogenDx (Calgary, AB) and Mayo Clinic Laboratories (Rochester, MN, USA) between 2016 and 2020 was undertaken, with chart review of paper and digital records of patients with discordant results. All results in Alberta were obtained by data retrieval by Alberta Precision Laboratories (APL), and chart review was performed using Allscripts Sunrise™ Clinical Manager and Alberta Netcare. All data were collected, reviewed, and stored following confidentiality requirements of Good Clinical Practice guidelines. Informed consent was waived by approving bodies given the QI nature of the project and was deemed not to be feasible (Alberta Government Health Information Act, Section 50).
Laboratories and assays
This analysis compared IgG anti-AQP4 and IgG1 anti-MOG CBA results at MitogenDx and Mayo Clinic Laboratories. MitogenDx is an accredited in vitro diagnostic laboratory in Calgary, Alberta, which specializes in biomarker assays for the diagnosis of autoinflammatory disorders. MitogenDx uses the Euroimmun (Lübeck, Germany) fixed cell-based immunoassay (CBA), which allows for qualitative detection by immunofluorescence microscopy of IgG antibodies directed against AQP4 and MOG. The CBA has been in use for AQP4 since 2009, and for MOG since June 2017. Interpretation of the assay result is based on the presence of an immunofluorescent signal at various dilutions of patient sera: negative, weak positive (at 1:10 end point dilution), medium positive (at 1:100 end point dilution), or high positive (at 1:1000 end point dilution). For the purposes of our study, “negative result” refers to a result falling within the normal reference range set by the individual laboratories. Specific details regarding how the assays are performed are available from the manufacturer and laboratory website (https://mitogendx.com/diagnostic-tests/neuromyelitis-optica-spectrum-disorder-nmosd-test-anti%E2%80%90aquaporin-4-mog/). This is typically the first-line anti-AQP4/MOG autoantibody investigation performed in Alberta and at many Canadian centres for patients with suspected NMOSD or MOGAD.
Mayo Clinic Laboratories is almost always the outsourced testing laboratory used for patients seen in Calgary with suspected NMOSD/MOGAD. Mayo Clinic Laboratories offers assays for detection of IgG anti-AQP4 and IgG1 anti-MOG and uses a live CBA methodology based on flow cytometry (FACS). Specific details regarding the assay procedure can be found on the laboratory website (https://www.mayocliniclabs.com/test-catalog). Based on published literature, the assays have been reported to be accurate and have previously been compared to assays such as Euroimmun with high rates of agreement. Reference Waters, Pittock and Bennett18–Reference Prain, Woodhall and Vincent20
Data collection and selection of paired subject tests
APL maintains a database of all tests ordered and performed within the provincial health organization (AHS). Using this resource, we retrospectively identified all patients seen by Calgary neurological services who underwent testing for anti-AQP4 or anti-MOG autoantibodies from January 2016 to July 2020. For feasibility and to focus on our QI question regarding concordance between MitogenDx and Mayo Clinic Laboratories, only patients with testing of anti-AQP4/MOG autoantibodies at both MitogenDx and Mayo Clinic Laboratories were included for further study. All tests labeled as “NMO/AQP4/MOG” were considered eligible. The total number of tests we started with, and the number at each laboratory are reported in the Results section.
We next paired each Mayo Clinic Laboratory test result for anti-AQP4 and anti-MOG to a corresponding result from MitogenDx, termed the “best temporal match.” The best temporal match was the first available Mayo Clinic test entry performed after (or in rare cases, concurrently with) MitogenDx testing, to reflect real-world practice at our centre and address the QI question. Since there were several patients who were tested multiple times at MitogenDx and fewer times at Mayo Clinic Laboratories, we carefully reviewed each match based on time of testing to ensure test pairs would not appear spuriously discordant. For all autoantibody testing, we excluded pairs of tests where the precise date of testing was not available, or where the interval between tests was more than 365 days. This was done in an attempt to avoid misclassifying test pairs as “discordant” when the discordance could be explained by spontaneous seroreversion over time, as has been observed with MOGAD. Supporting the use of this time interval, a population-based study of serial anti-MOG serologies in over 400 children found the median time to serorevert from positive to negative was 365 days. Reference Waters, Fadda and Woodhall24 We determined that including assay pairs up to 365 days apart was of sufficient duration to capture cases in which clinicians were outsourcing tests for diagnostic confirmation in routine clinical care. In the case of anti-MOG autoantibodies, we planned to scrutinize discordant pairs with positive local and negative outsourced results from 183 to 365 days to reflect that some clinicians check for seroreversion as early as 6 months (183 days). All included pairs were of serum samples (one pair where CSF was mistakenly compared with serum was excluded).
Review of clinical and demographic information
Each identified test pair was categorized as concordant or discordant. Only patients with discordant results underwent chart review, because the scope of our QI project and approvals required review of confidential data to be limited and focused. In this context, review of discordant cases was prioritized to better understand the impact of these often-vexing cases on clinician assay ordering practices. Furthermore, detailed clinical review of all cases in the sample (with hundreds of concordant pairs) was deemed unfeasible. Chart data collected included age at index presentation, sex, ethnicity (where documented), clinical syndrome, description of clinical course, neuraxial magnetic resonance imaging (MRI) findings, anti-AQP4 and anti-MOG autoantibody test dates and results (at each laboratory), test timing with respect to treatment, acute relapse treatment, chronic immunomodulatory treatment, Expanded Disability Status Scale (EDSS), visual acuity, and other relevant symptoms. For discordant cases, we determined the pre- and post-outsourced test diagnosis and influence of the result on treatment.
Analysis
The interlaboratory, within-patient (subject) concordance rate was determined for anti-AQP4 and anti-MOG assays by using the identified valid test pairs (Figure 1). The median and range for the number of days between tests in each pair (testing interval) were determined. Means and standard deviations were not normally distributed, so median and range are reported in the Results section instead. The proportion of concordant test results for each of anti-AQP4 and anti-MOG were determined along with 95% confidence intervals (CIs). Cohen’s kappa coefficient was calculated to assess interlaboratory agreement for each of the anti-AQP4 and anti-MOG assays. All calculations were done using Microsoft Excel™ version 16.6. For the discordant cases which underwent chart review, each case was classified according to how the clinical phenotype corresponded to the findings on anti-AQP4 and anti-MOG autoantibody testing. Due to the scope of our QI approvals, this study was not designed to evaluate assay characteristics such as sensitivity and specificity, nor positive/negative predictive values in our population.
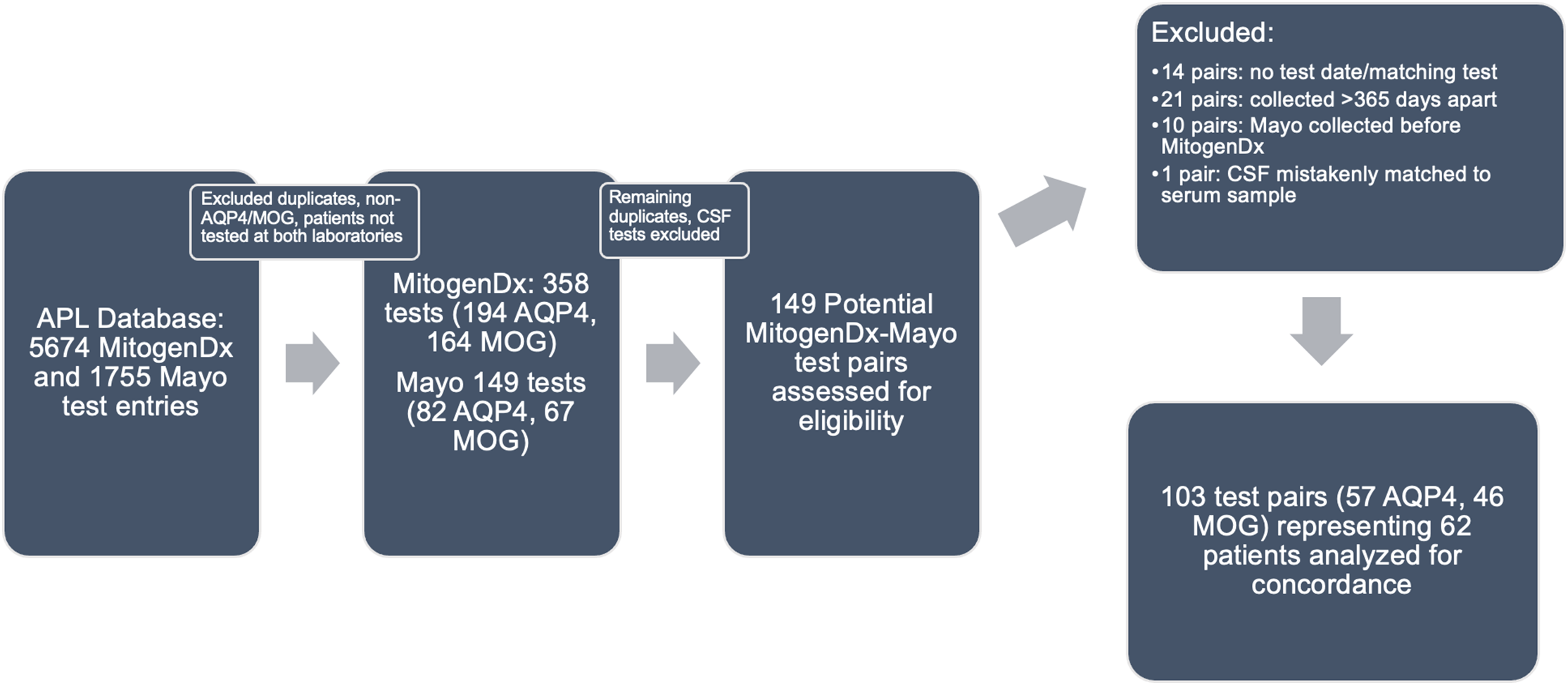
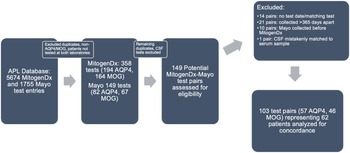
Figure 1: Paired test selection (2016–2020). An iterative process of removing duplicates, identifying true serum AQP4/MOG tests, and generating pairs was done as described in the Methods. Abbreviations: APL–Alberta Precision Laboratories; Mayo–Mayo Clinic Laboratories; AQP4–test for anti-aquaporin-4 autoantibodies; MOG–test for anti-myelin oligodendrocyte glycoprotein autoantibodies.
Results
Results of primary data retrieval
In the initial data retrieval, there were a total of 5,674 unique test records for MitogenDx (4,864 for anti-AQP4 autoantibodies with 68 positive results and 810 for anti-MOG autoantibodies with 50 positive results) and 1,755 for Mayo Clinic Laboratories. After selecting for tests confirmed to be for anti-AQP4/MOG and representing only patients evaluated at both laboratories, there were 358 unique test entries from MitogenDx (194 AQP4, 164 MOG) and 149 entries from Mayo Clinic Laboratories (82 AQP4, 67 MOG). Among MitogenDx anti-AQP4 samples there were 4 positives (3 high positive and 1 weak positive), 155 negatives, and 35 unretrievable results. MitogenDx anti-MOG results featured 19 positives (4 high positive, 6 medium positive, and 9 weak positive). Thresholds for high, medium, and weak positive at MitogenDx are as defined in the Methods. For the 149 Mayo Clinic entries, there were 82 anti-AQP4 and 67 anti-MOG samples. Mayo Clinic anti-AQP4 results featured 6 positives (titres ranging from 1:10 to 1:10,000) and 76 negatives. Mayo Clinic anti-MOG tests resulted in 8 positives (titres ranging from 1:20 to 1:1000) and 59 negatives. Of note, only 12% of patients tested for anti-AQP4, and 9% of patients tested for anti-MOG had repeat testing locally prior to outsourced Mayo Clinic Laboratory testing.
Concordance analysis of valid interlaboratory test pairs
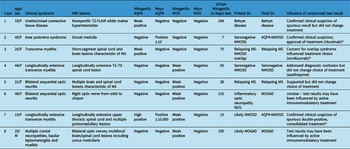
We next generated pairs of tests for our concordance analysis as outlined in the Methods and Figure 1. This resulted in 103 valid interlaboratory test pairs (57 AQP4 and 46 MOG) representing 62 unique patients. Fifty-four of 57 (94.7%, 95% CI [88.9, 100]) anti-AQP4 test pairs were concordant between laboratories; 3 pairs were discordant (Table 1). The kappa coefficient for anti-AQP4 was 0.88, representing superb interlaboratory reliability. A median of 53 days (range 0–336) separated concordant anti-AQP4 pairs and 70 days (range 7–194) separated discordant anti-AQP4 pairs. Two of the discordant anti-AQP4 cases featured weak positive anti-AQP4 results locally (Tables 2 and 3; cases 1 and 3). Neither of these patients had phenotypes consistent with NMOSD and were ultimately diagnosed with Behçet’s disease and relapsing MS, respectively. Neither patient was on immunotherapy at the time of MitogenDx testing. One of these patients was on azathioprine at the time of Mayo Clinic testing; the other was on glatiramer acetate, which was unlikely to affect serostatus (Table 3). A single presumed false-negative local MitogenDx anti-AQP4 result in prototypical NMOSD was the last discordant case (Table 3, case 2). This patient did not receive immunotherapy prior to their initial test.
Table 1: Concordance breakdown by autoantibody. Concordance rates are displayed as a percentage of the total number of test pairs for each autoantibody along with 95% CIs (Microsoft Excel™ version 16.6). Median days separating MitogenDx from the matched Mayo clinic test and corresponding range are shown. Cohen’s kappa coefficient representing interlaboratory reliability was near-perfect for AQP4 and moderate for MOG
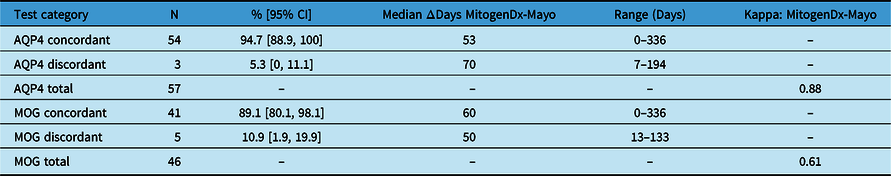
AQP4=anti-aquaporin-4 autoantibody assay; MOG=anti-myelin oligodendrocyte glycoprotein autoantibody assay; N=total number; ΔDays=number of days separating the MitogenDx test in the pair from the Mayo test; Kappa=Cohen’s kappa coefficient.
Table 2: Summary of interlaboratory discordant cases. Age specified is at index presentation. All anti-AQP4 and anti-MOG tests were done on serum. Please see Methods for definitions of weak positive, high positive, etc
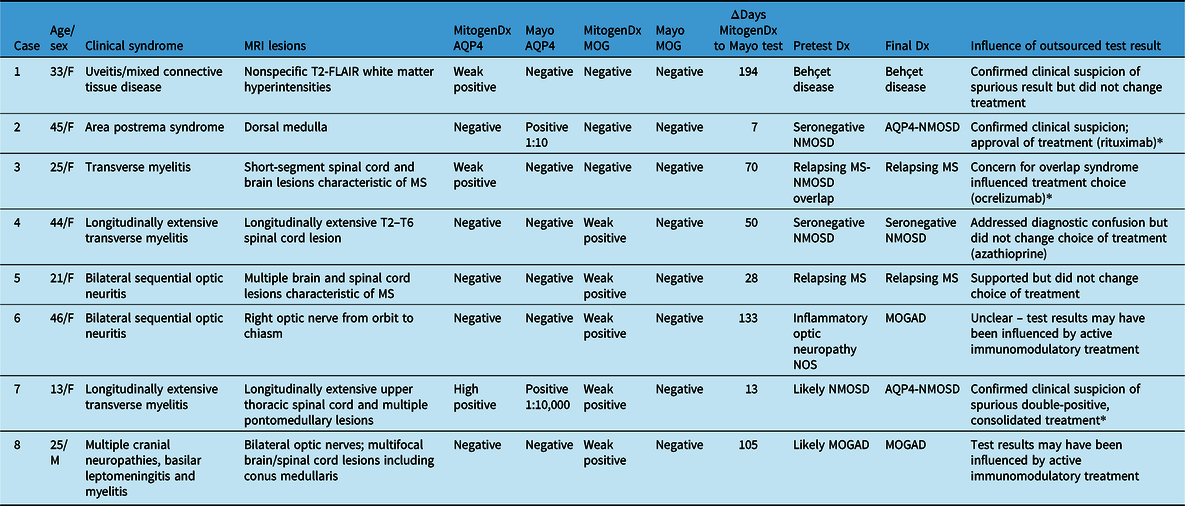
MRI=magnetic resonance imaging; Dx=diagnosis; AQP4=anti-aquaporin-4 autoantibodies; MOG=anti-myelin oligodendrocyte glycoprotein autoantibodies; FACS=fluorescence-associated cell sorting assay; NOS=not otherwise specified; NMOSD=neuromyelitis optica spectrum disorder; MS=multiple sclerosis; MOGAD=MOG antibody-associated disease; CNS=central nervous system.
* indicates the cases where the result of outsourced testing altered management.
Table 3: Immunotherapy in discordant cases. Cases are numbered as in Table 2. Immunomodulatory therapy at the time of testing at each laboratory and the number of days between tests are indicated. All tests were on serum; definitions of weak positive can be found in Methods.
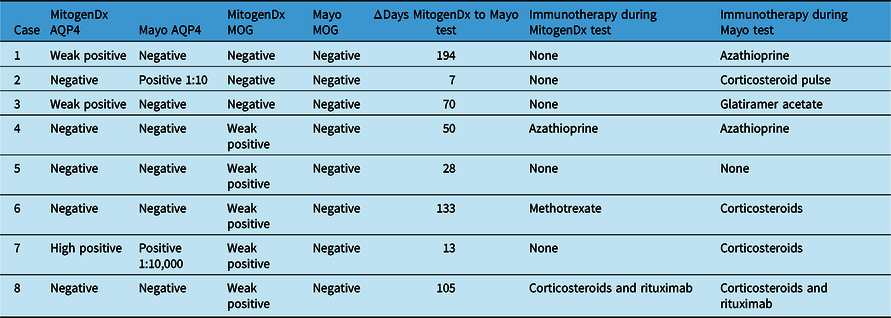
Dx=diagnosis; AQP4=anti-aquaporin-4 autoantibodies; MOG=anti-myelin oligodendrocyte glycoprotein autoantibodies.
Forty-one of 46 (89.1%, 95% CI [80.1, 98.1]) anti-MOG test pairs were concordant between laboratories (Table 1). Kappa coefficient was 0.61, consistent with moderate interlaboratory reliability for anti-MOG assays. The five discordant cases were all associated with weak positive local results that were negative on subsequent Mayo Clinic Laboratory testing (Table 2). A median of 60 days (range 0–336) separated concordant anti-MOG pairs and 50 days (range 13–133) separated discordant anti-MOG pairs.
Among the five patients with weak positive anti-MOG results locally and negative outsourced test results, three had phenotypes consistent with MOGAD (bilateral optic neuritis, longitudinally extensive myelitis, and encephalomyelitis with leptomeningeal basilar meningitis), one was diagnosed with relapsing MS, and one was later found to have anti-AQP4 seropositive NMOSD. As outlined in Table 3, three of the five patients were prescribed immunotherapy at the time of MitogenDx testing, and four of five were prescribed immunotherapy at the time of Mayo Clinic testing.
Outsourced testing impacted management in three of eight total discordant cases (Table 2, final column). In two of these cases, clarification was achieved regarding the anti-AQP4 result and in one case regarding the anti-MOG result. In these cases, results supported the selection of specific long-term immunomodulatory treatment that required application to a government-based financial support program. For example, one patient who presented with area postrema syndrome initially tested negative for anti-AQP4 autoantibodies at MitogenDx. Subsequent testing at Mayo Clinic Laboratories confirmed ongoing clinical suspicion of anti-AQP4 seropositivity and enabled access to rituximab.
To ensure our selection process did not unduly exclude discordant test pairs, we reviewed interlaboratory test pairs collected >1 year apart, and no additional discordant cases were found. Only one of the discordant cases (Table 2, case 1) featured a MitogenDx-Mayo Clinic anti-AQP4 test pair collected >6 months apart. None of the discordant pairs represented anti-MOG testing completed 6–12 months apart. Given low numbers of discordant cases, further statistical analysis of demographic and ancillary clinical features was not performed.
Discussion
Our QI project found MitogenDx and Mayo Clinic Laboratories CBAs to be highly concordant in detecting anti-AQP4 and anti-MOG autoantibodies in patients seen by Calgary neurological services. We observed excellent interlaboratory reliability for anti-AQP4 autoantibody assays (kappa 0.88), whereas anti-MOG assays were more likely to be associated with discordance (kappa 0.61). Outsourced testing influenced management in three of eight discordant cases, representing a small minority of the overall tested population. This suggests that the local practice of outsourcing tests (and cost associated with this) could be modified and reduced. Proposed algorithms for testing generated based on our results are shown in Figures 2 and 3. These algorithms were developed based on consensus discussions between the authors of this study (consisting of two NMOSD/MOGAD experts in Canada, KA and JMB).
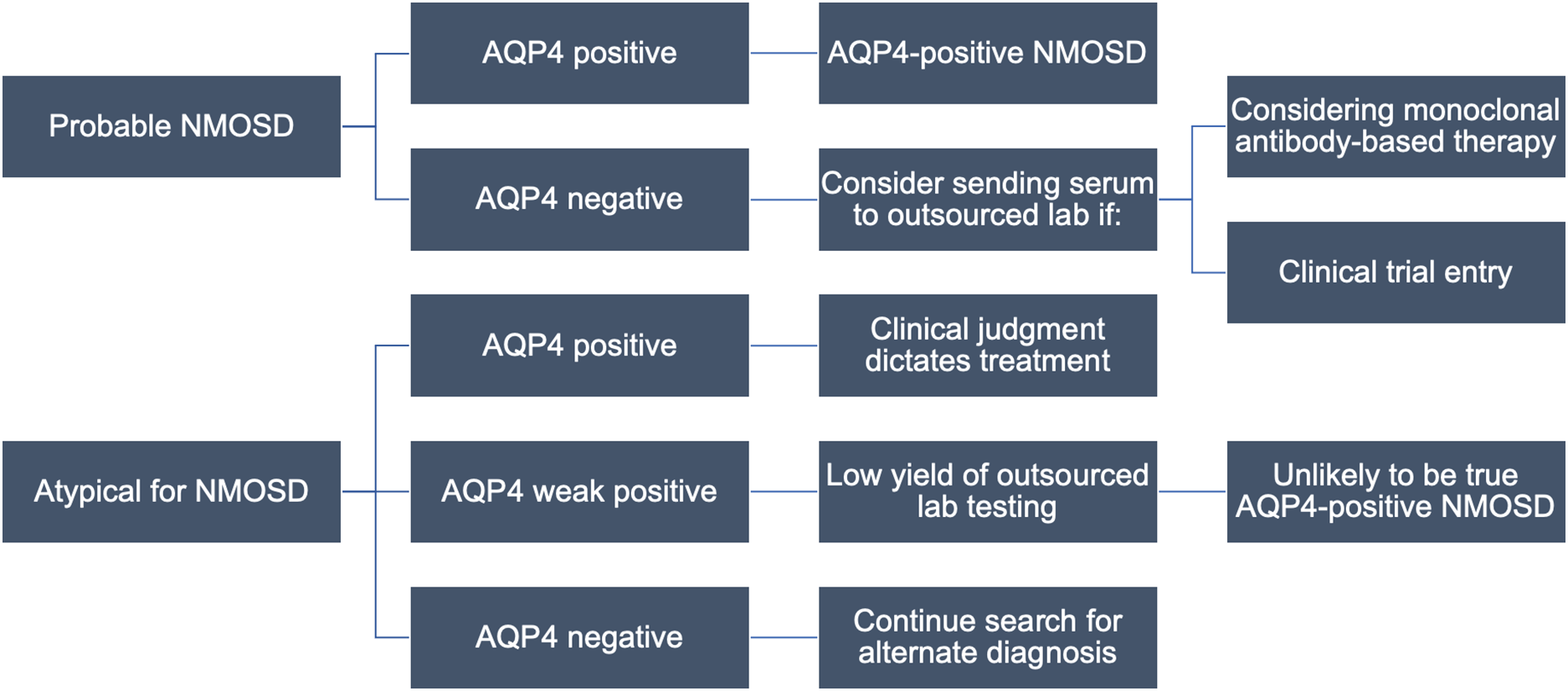
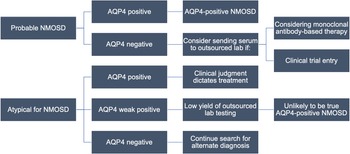
Figure 2: Proposed local testing algorithm for NMOSD. The approach to testing begins with the clinical phenotype and assumes initial testing at MitogenDx. Cases which may still benefit from outsourced testing are outlined. Abbreviations: AQP4–aquaporin-4; NMOSD–neuromyelitis optica spectrum disorder.
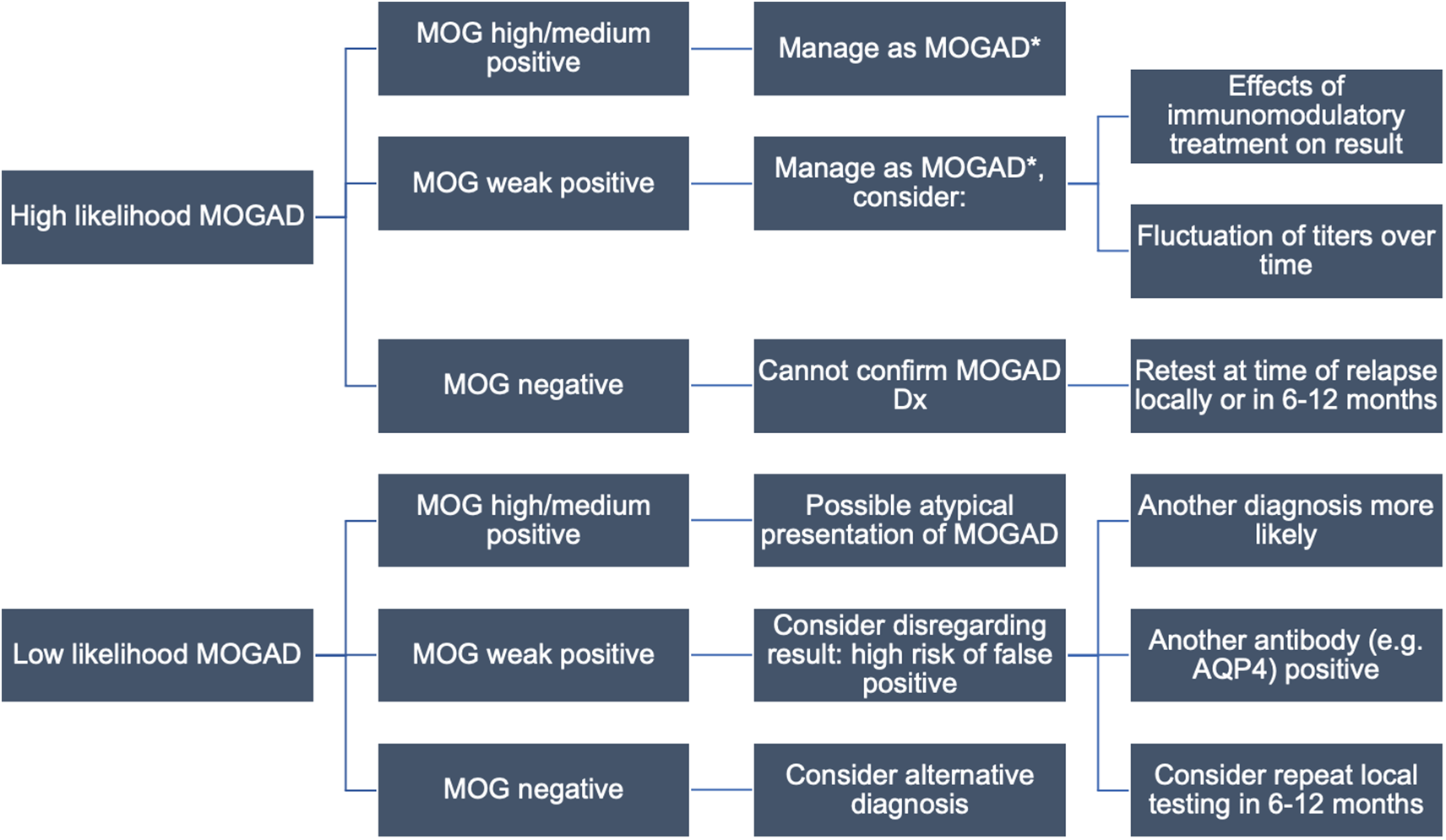
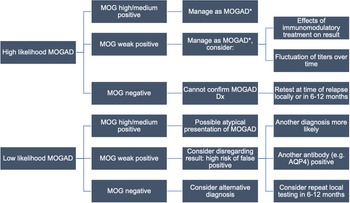
Figure 3: Proposed local testing algorithm for MOGAD. The approach to testing begins with the clinical phenotype and assumes initial testing at MitogenDx. Interpretation of autoantibody results and follow-up testing depends on the clinical context. Caution with weakly positive results or any tests done while on immunomodulatory treatment is advised. *No consensus treatment guidelines are available. Abbreviations: AQP4–aquaporin-4; MOG–myelin oligodendrocyte glycoprotein; MOGAD–MOG antibody-associated disease.
This study identifies subgroups for whom confirmatory testing may be indicated. Patients with prototypical NMOSD who have a “negative” (within normal reference range) test for anti-AQP4 locally may benefit from outsourced confirmation of anti-AQP4 results if treatment with serostatus-restricted monoclonal antibodies is necessary. This may be especially true for patients with a higher pretest probability of NMOSD (e.g., female, older, Asian, or Afro-Caribbean ethnicity, classic presentations), Reference Stratos, Lee and Dai25,Reference Papp, Magyari and Aktas26 who were associated with two of three discordant anti-AQP4 test pairs and one of five discordant anti-MOG pairs. All three of these cases were females of non-White ethnicity, two of whom where >40 years of age at index presentation. Two of them presented with longitudinally extensive transverse myelitis and one with area postrema syndrome, all consistent with NMOSD. Reassuringly, we identified only one case with a suspected false-negative anti-AQP4 result at the local laboratory (whose presentation was highly consistent with NMOSD). This supports the position that outsourced testing for anti-AQP4 autoantibodies has a low yield, and candidates should be carefully scrutinized.
Conversely, no cases of local negative, Mayo Clinic positive anti-MOG autoantibody pairs were found, suggesting outsourced testing has limited value when local results are negative. Serum anti-MOG autoantibody titres are known to fluctuate over the disease course, in part due to certain forms of immunomodulatory treatment, and natural history in apparently monophasic disease in some patients (e.g., associated with ADEM). Reference Cobo-Calvo, Ruiz and Maillart7,Reference Jarius, Ruprecht and Kleiter27 Therefore, repeat local testing for anti-MOG autoantibodies at the time of clinical relapse prior to the use of acute immunomodulatory treatments (and if feasible, off any immune therapies) might be of higher yield.
Weak positive anti-MOG results at MitogenDx (negative at Mayo Clinic Laboratories) were responsible for all five anti-MOG discordances. A complete analysis of patients with any anti-MOG weak positive result irrespective of concordance was outside the scope of our QI (ethical) approvals, although a possible explanation for weak (borderline) positive anti-MOG being overrepresented among discordant cases may be related to cutoffs being set too low within the current reference range used at MitogenDx. One of these cases also had a high positive anti-AQP4 titre at the same laboratory and was clinically consistent with the diagnosis of anti-AQP4 seropositive NMOSD (Table 2, row 7). Outsourced testing reinforced clinical suspicion that the weak positive anti-MOG result was a distractor (false-positive). This is consistent with literature showing that “double seropositive” patients are rare, and likely due to false-positive anti-MOG results, particularly when anti-AQP4 titres are high. Reference Kunchok, Chen and McKeon28 Three of the remaining four weak positive MitogenDx anti-MOG discordant cases (negative at Mayo Clinic) were clinically compatible with MOGAD. It is possible that this could be due to fluctuations in anti-MOG autoantibody titres or in response to immunotherapy.
Based on previous studies, it is known that low or borderline positive anti-MOG results are more likely to be unreliable and produce discordance between reputable laboratories. Reference Reindl, Schanda and Woodhall23 The specificity of low positive anti-MOG titres for MOGAD has also been questioned by others in the field, who have found that this autoantibody can be detected at low titres in patients with other immune-mediated (e.g., encephalitis, MS, and parainfectious) and nonimmune disorders (e.g., glioblastoma) that cause CNS damage. Reference Levy, Yeh and Hawkes29,Reference Manzano, McEntire and Martinez-Lage30 Persistent anti-MOG seropositivity correlates with a multiphasic disease course and may be an indication to start chronic immunomodulatory therapy, so it is important to identify such patients. Reference Lopez-Chiriboga, Majed and Fryer31,Reference Chen, Flanagan and Bhatti32 Overall, our study reinforces the lesson that clinical judgment is crucial for determining which patients should be evaluated for anti-AQP4/MOG autoantibodies and assessing the relevance of the result for diagnosis and management (Figures 2 and 3).
A strength of our study is the inclusion of all relevant tests performed for patients seen in Calgary over several years. Due to the centralized database housed at APL, we can be assured that no anti-AQP4 or anti-MOG tests were missed over the epoch studied apart from rare tests that did not have retrievable results. Our study also demonstrated the benefits of collaboration between clinicians, laboratory scientists, and laboratory administrators with the goal of improving patient outcomes and resource management.
Our study has limitations. The retrospective and real-world QI design did not allow us to predetermine which patients would be tested for anti-AQP4 and anti-MOG autoantibodies. There was likely variable pretest probability of NMOSD and MOGAD depending on the individual practice of the ordering clinician, which may have decreased the overall likelihood of a positive result at either laboratory, and might have influenced the concordance analysis. By design, we only sought results for patients who underwent both local and outsourced testing. Therefore, our sample likely overrepresents patients in which diagnostic uncertainty exists. We did not collect data from patients with concordant results, since it was outside the scope of our QI design, and not feasible. Therefore, we were not able to study assay performance by comprehensively defining true positives and true negatives to generate sensitivity and specificity metrics. Secondly, our local project parameters do not allow these results to be broadly generalized; however, we note that multiple centres in Canada also use MitogenDx and may use analogous testing strategies. Additionally, with respect to anti-MOG autoantibody discordance, it is possible that factors other than test accuracy, such as natural fluctuations or the effect of treatment, could have impacted results. Finally, a formal cost-effectiveness analysis was not performed. At the extreme end of the spectrum, if we assume that all 95 concordant tests were not strictly necessary, this would amount to over $100,000 CAD in additional cost over the 2016–2020 epoch, based on costs at the time of writing.
Future studies should involve collaboration with local stakeholders to refine the testing strategy for patients with suspected NMOSD and MOGAD based on our findings. In particular, the MitogenDx laboratory will be examining whether weak positive anti-MOG results could be explained by modifiable assay characteristics such as recalibration of the reference range and cutoffs. Weak positive results should be reported to clinicians with an emphasis on cautious interpretation. Similar testing strategies at our centre are used for patients with suspected antibody-mediated encephalitis and other autoimmune neurological syndromes. Thus, our QI model used to interrogate anti-AQP4 and anti-MOG assays could be applied to evaluate the merit of testing practices for autoantibodies in other neurological diseases. Future prospective study and iterative QI will help decide whether a more focused testing strategy alters the cost and quality of patient care at our institution.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2022.324.
Acknowledgments
We would like to acknowledge the technical services of Ms. Haiyan Hou and Meifeng Zhang (MitogenDx) along with technologists and representatives at Alberta Precision Laboratories, Alberta Health Services, for their assistance in collecting the data necessary to perform this QI project.
Statement of Authorship
JDK helped with conceptualization of the project, conducted data analysis, and wrote the manuscript. MJF provided expert opinion regarding the methodology, laboratory assays, and data analysis and revised the manuscript. KA was involved in project conceptualization, supervision, and manuscript revision. JMB obtained necessary approvals, conceptualized and supervised the project, supervised data collection/analysis, and wrote and revised the manuscript.
Disclosures
This research was supported by unrestricted funds from the University of Calgary and MS Society of Canada to Dr. Jodie M. Burton (JMB).
JDK has nothing to disclose.
KA has received honoraria for serving on the advisory boards at Biogen, Bristol Myers Squibb, EMD Serono, Novartis, Roche, Sanofi Genzyme, and support for conference attendance by Biogen.
MJF is the medical director of MitogenDx and has received honoraria from Werfen (San Diego/Barcelona).
JMB has received honoraria for serving on the advisory board and as a webinar presenter for Roche and providing educational activities for Biogen, Novartis, and Alexion. Educational and consulting activities were provided for PRIME Education, LLC, with no honoraria received.