Pituitary edema (PA) and Rathke’s cleft cyst (RCC) are both common and benign sellar lesions; however, they rarely occur together. In a large study series involving 782 patients with known PA, the rate of concomitant RCC was 0.51%.Reference Noh, Ahn, Lee and Kim 1 According to a recent report, eight cases (1.46%) with dual sellar pathology were histologically diagnosed among 548 cases with PA, and only two concomitant RCCs were identified (0.74%).Reference Koutourousiou, Kontogeorgos, Wesseling, Grotenhuis and Seretis 2 According to our review of the literature, most of the articles were case reports that lack long-term follow-up and comprehensive review. There is considerably less knowledge regarding PA concomitant with RCC, and the clinicopathological characteristics have not been well described. Here, we report 15 patient cases with the aim of analyzing the clinical, radiological, surgical, histological, and prognostic characteristics of these coexisting lesions. The analysis is presented with a review of the literature to systematically investigate the clinicopathological features of these collision lesions.
Methods
This study was approved by the Institutional Review Board of Beijing Tiantan Hospital. We retrospectively studied the medical histories, physical examination results, imaging results, operative findings, and histological examinations of the 15 patients with PA and concomitant RCC. The RCCs were grossly unruptured. All 15 patients were treated at Beijing Tiantan Hospital between 2009 and 2016. All patients were preoperatively and postoperatively evaluated with endocrine testing and magnetic resonance imaging (MRI). The endocrine testing includes prolactin (PRL), growth hormone (GH), insulin-like growth factor, follicle-stimulating hormone, luteinizing hormone, adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone, and so on. The resected tumor specimens were evaluated by experienced pathologists to make a histological diagnosis to ensure a functional classification of the PAs could be performed. The follow-up results included changes in clinical symptoms, enhanced MRI scan results, and endocrine testing.
For our literature review, an advanced search in PubMed for English articles was carried out. The key words used were “adenoma” and “Rathke’s,” which were entered into the title/abstract field to search the related literature as comprehensively as possible. The search yielded 128 results from 1981 to 2016; among these results, 23 English-language articles were identified that reported on patients involving a PA associated with RCC.
Results
Preoperative History
The clinical data for the 15 patients are available in Table 1. The patients were eight males and seven females. Their mean age was 48.1±12.4 years (range, 20-67 years). Headache and visual disabilities were the most common symptoms. The clinical manifestations of the functional PAs depended on the hormone secreted and could present as a menstrual disorder, galactorrhea, or acromegaly. One patient was diagnosed with tumor recurrence; she had had transsphenoidal surgery 1 year previous. There were no hypothalamic dysfunction or increased intracranial pressure reports in this group. The course of illness ranged from 15 days to 10 years.
Table 1 Clinical data of the 15 patients with PA associated with RCC
F=female; FSH=follicle-stimulating hormone; IGF=insulin-like growth factor; IS=intrasellar; LH=luteinizing hormone; M=male; PS=parasellar; SS=suprasellar; TS=transsphenoidal.
Radiological Findings
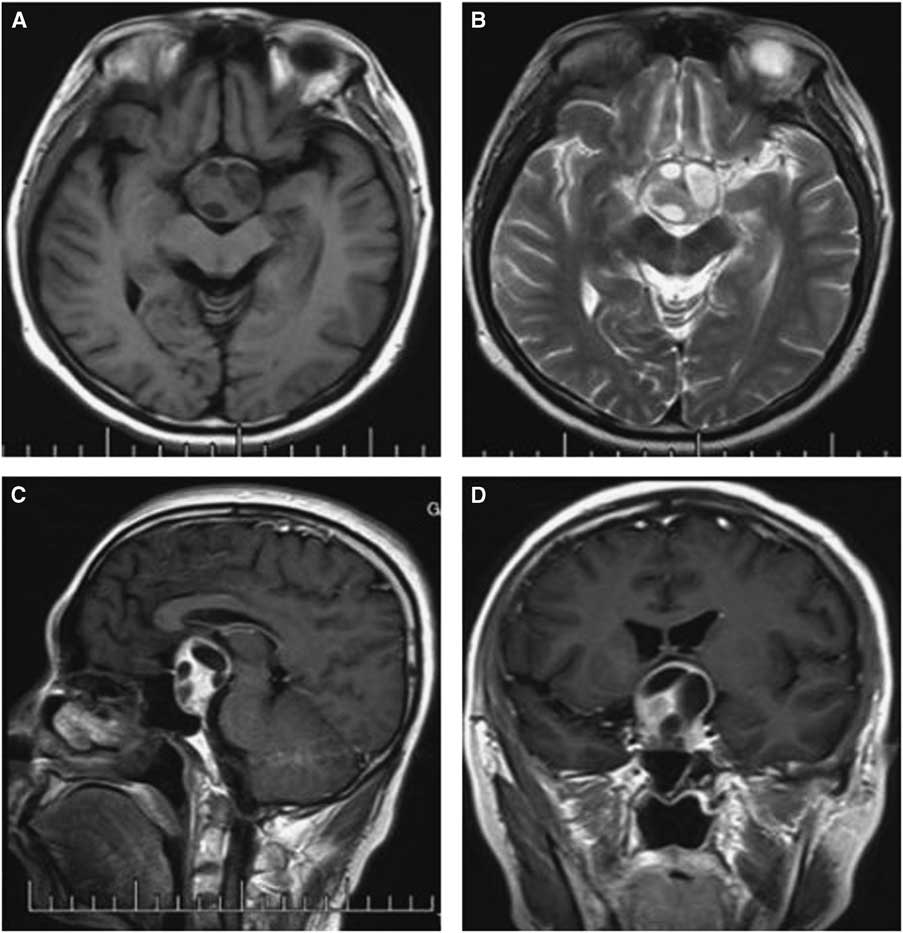
Computed tomography and MRI were performed for each patient preoperatively. Of the PAs, two were microadenomas diagnosed on dynamic contrast-enhanced scan of the sellar region with MRI. The others were macroadenomas. The tumors were mainly located in the intra- and suprasellar areas, except for four cases that involved the parasellar region. Among these cases, two were grade 3 on the Knops scale, whereas the other two were grade 4. The typical imaging of PA associated with RCC was that a cyst-like signal with no contrast enhancement was indicated on MRI with an obvious PA, and the cyst was usually enclosed by the PA or adjacent to the adenoma, as shown in case 3 (Figure 1). However, it was occasionally difficult to identify the two different signal intensities between the PA and RCC, as indicated in case 1 (Figure 2). The 15 patients were all initially diagnosed with PAs or PAs with cystic change or apoplexy of PAs.
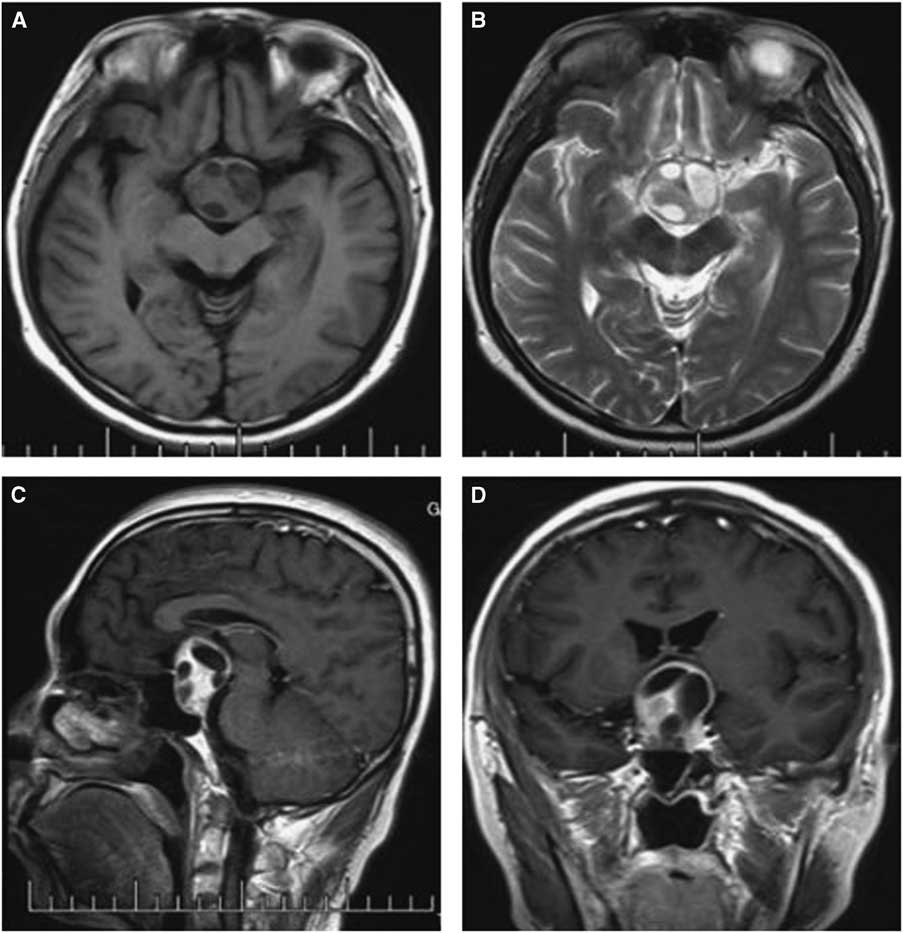
Figure 1 Preoperative and postoperative MRI scans of case 3. Axial T2-weighted (A) images showing an isointense mass in the sellar region with multicystic lesions inside; Contrast-enhanced sagittal (B) and coronal (C) images show obvious enhancement of the solid mass and no changes in the cysts, with the tumor extending into the suprasellar area. The postoperative images show the tumor was totally removed and there was a hemorrhage at the site of the operation (D-F).
Figure 2 Preoperative and postoperative MRI scans of case 1. Axial T2-weighted (A) images revealing uneven signal intensity in the pituitary measuring approximately 4 mm in greatest diameter. (B) A contrast-enhanced sagittal image showing obvious enhancement of the pituitary, but identifying two different entities was difficult. (C) Dynamic contrast-enhanced image demonstrating a delayed-enhancement region on the left of the pituitary, with the pituitary stalk displaced slightly to the right; The postoperative images showed the tumor was resected completely (D-F).
Surgical Results
Based on the clinical and radiological findings, a transsphenoidal approach was adopted for all patients except one, who underwent right frontal craniotomy. Complete removal was achieved in nine cases, whereas subtotal resection was achieved in five and partial removal in one. During surgery, clear yellow fluid was observed in three patients and mucinous and colloidal materials were encountered in five patients.
After surgery, the patients’ headaches were relieved and their vision improved. A computed tomography scan on the first day after surgery indicated that there was a hemorrhage at the site of the operation in cases 3 and 15. Conservative management and surgical clearance was adopted, respectively. The hematoma was stable and partially absorbed at the time of discharge. Postoperative endocrine evaluation indicated that the patients’ PRL and GH levels returned to the normal range or decreased compared with preoperative levels. Additionally, some of the patients demonstrated mild or moderate thyroid dysfunction and transient electrolyte disturbance and were thus given symptomatic and supportive treatment.
Pathological Findings
Microscopically, the tumor contained two components. The first component was monomorphic cells with round or oval nuclei containing stippled chromatin. The second component showed fragments of a cyst wall lined by simple cuboidal or pseudostratified ciliated columnar epithelium; the lumen of the cyst contained an abundance of myxoid materials (Figure 3A). Immunohistochemical analysis demonstrated positive results for PRL and GH/PRL in three patients (Figure 3B-C). A positive result for GH staining was indicated in one patient. There was no obvious staining in the other five cases, which were diagnosed as nonfunctional PAs (NFPAs) associated with RCC. Additionally, positive expression of cytokeratin in 8/18 cases was observed.
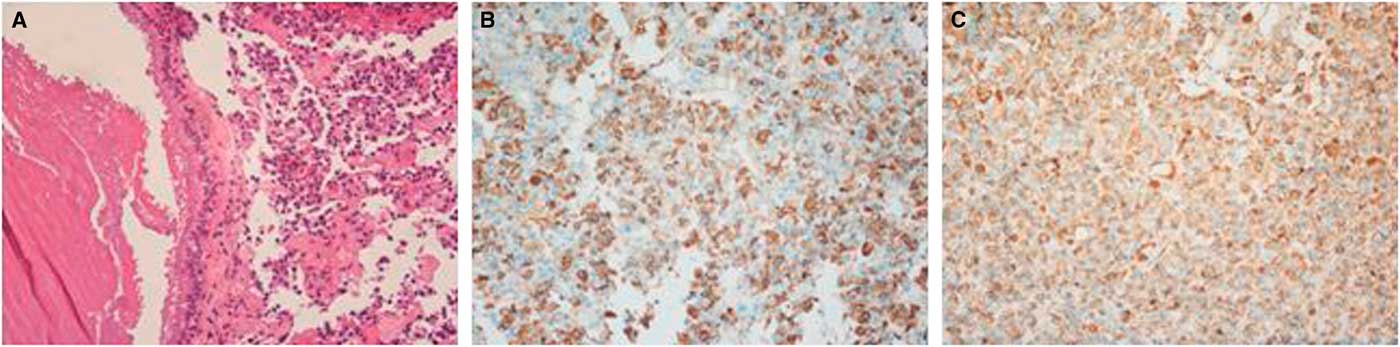
Figure 3 Hematoxylin-eosin and immunohistochemical findings for case 12. (A) Clusters of uniform PA cells with mucin-filled cyst. The cyst was lined by ciliated columnar epithelium (×200). (B) Immunostaining of GH was positive in the adenoma cells (×200). (C) PRL staining of adenoma cells showed strong reactivity (×200).
Follow-up
Fourteen patients continued their follow-up; one patient was lost to follow-up. The median follow-up period was 27.6±16.6 months (4-53 months). Among the 14 patients, 13 had no obvious abnormality of endocrine testing, and their MRI examinations showed no evidence of recurrence. Case 15 suffered from tumor recurrence and increased PRL level during the follow-up. Case 4’s menses returned but was still irregular. Case 5 did not undergo MRI examination and had no obvious symptoms. In case 6, the patient suffered from cerebral infarction 15 days after discharge with mild neurological defects.
Literature Analysis
The 23 reports published from 1985 to 2016 in PubMed were reviewed, and only 59 cases of PA and concomitant RCC were included. The clinicopathological characteristics of the patients are summarized in Table 2. There were 17 males and 24 females, and they varied in age from 16 to 76 years (median, 42 years). The most common complaints varied from patient to patient and can be classified as endocrine symptoms and mass effect generally. From our review, the former occurred more often than the latter.
Table 2 Clinical data of the reported cases with PA associated with RCC
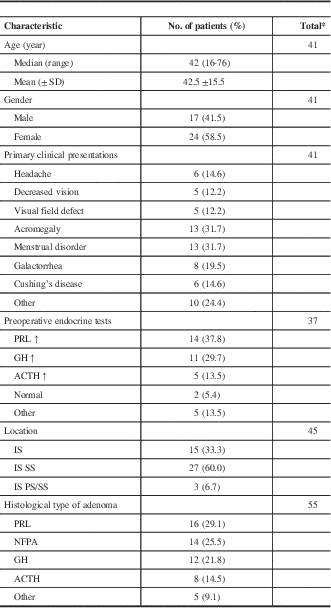
* Only patients with required data were included.
SD=standard deviation.
Most of the tumors were located at the intra- and suprasellar region (93.3%), as observed on MRI. Occasionally, the tumor invaded into the parasellar area (6.7%). All of the patients underwent surgical resection via a transsphenoidal approach, except for four patients who required right frontal craniotomy. Among the 55 patients with histological data, 16 patients had prolactin-producing adenomas, 14 had NFPAs, 12 had GH-secreting adenomas, 8 had ACTH-secreting adenomas, and 2 had mixed adenomas. Additionally, three patients were classified using traditional hematoxylin-eosin staining. Surgical results were available for 32 patients. Most of the patients recovered uneventfully, and their hormonal imbalances were corrected. Three patients experienced hypopituitarism after their operation, and hormone replacement therapy was administered. Three patients experienced diabetes insipidus, which was well controlled with medication. One patient suffered from cerebrospinal fluid rhinorrhea, which subsided after lumbar drainage combined with transsphenoidal sellar floor repair. There was no long-term follow-up.
Discussion
The coexistence of PA with RCC has rarely been reported and is seldom encountered in clinical practice. To our knowledge, only 59 patients have been identified among 23 English articles.Reference Noh, Ahn, Lee and Kim 1 - Reference Gao, An and Huang 23 According to our review, there was a slight female predominance to the co-incidence of these lesions. The male-to-female ratio was approximately 0.7:1; however, there were more male patients than female patients in our case series. This may be the result of a limited number of patients. The clinical manifestations were mainly dependent on the hormone secreted and the size of the tumor. Endocrine symptoms such as amenorrhea, galactorrhea, acromegaly, and Cushing’s disease were usually caused by PAs. Further, with increasing tumor size, compressive signs or symptoms appeared, including headache, visual diminution, vision field defects, and so on. The literature review indicates that the endocrine symptoms occur more often than compressive symptoms. This is different from our patients. In our series, many cases had macroadenoma or even giant adenoma associated with RCC with supra- or parasellar extension, which contributes to mass effect.
A preoperative diagnosis of PA associated with RCC is difficult because most cases are clinically and radiologically similar to PAs; this is true especially for microadenoma of the pituitary and atypical cysts. Most of the diagnoses were based on histological results. In our group, two cases were diagnosed as microadenomas of the pituitary associated with RCC. The diagnosis of microadenoma itself is relatively difficult, and even MRI scans may yield negative results. The diagnosis depends on the combination of clinical symptoms, imaging results, and endocrine testing to achieve a comprehensive determination. Thus, the diagnosis of microadenoma of PA associated with RCC is more challenging. It is usually difficult to distinguish the two lesions on MRI scans. RCC may have a variable imaging appearance on MRI images depending on the cyst composition.Reference Valassi, Biller, Klibanski and Swearingen 24 The presence of mucinous fluid, hemosiderin deposition, and hemorrhage within the cyst contribute to the MRI characteristics.Reference Sumida, Migita, Tominaga, Iida and Kurisu 13 RCCs do not usually demonstrate contrast enhancement or show thin cyst wall enhancement, whereas PAs are characterized by homogeneous enhancement. When a cyst-like signal is observed on MRI scans in a patient with PA, the possibility of coexisting RCC should be considered.
Surgical resection and decompression represent the most effective treatment. Transsphenoidal surgery is performed for most reported cases, including our 14 patients. Both solid tissue and cystic material can be observed in some cases, and a probable diagnosis of PA associated with RCC can be made. However, any clear yellow fluid should not be mistaken for cerebrospinal fluid leakage caused by rupture of the sellar diaphragm. Compared with macroadenoma of the pituitary associated with RCC, microadenoma combined with RCC is more challenging both with respect to the preoperative diagnosis and the surgical resection. Because of the small tumor volume, the localization of a microadenoma coexisting with an RCC is difficult during the operation. Intraoperative navigation can help to determine the exposure range of the sellar floor and the location of the tumor, which is important for total resection and functional protection.Reference Hitier, Hibon, Candelier, Guarnieri, Moreau and Babin 25 Surgery can resolve or relieve patients’ symptoms to a great extent; however, the biochemical remission rates are greatly dependent on tumor size and depth of invasion.Reference Bader, Carter, Latchaw, Ellis, Wexler and Watson 5 Surgical results are satisfactory for most patients both in our series and literature, including our two cases with microadenoma of pituitary combined with RCC. After surgery, one patient’s PRL level returned to normal range and the other patient’s endocrine symptoms were relieved. These two patients were discharged uneventfully and recovered well. At times, it is not easy to achieve gross total resection if the tumor has invaded the cavernous sinus. In this case, radical removal is not recommended to avoid serious complications, and adjuvant therapy such as radiotherapy may be used. Intraoperative MRI scans can assist with complete resection and provide excellent information on these two different disease entities.Reference Radhakrishnan, Menon, Hingwala and Radhakrishnan 12 For the cyst itself, simple drainage of the contents with partial removal of the cyst wall is usually adequate and reduces the risk of pituitary-hypothalamic dysfunction, visual complications, and aseptic meningitis.Reference Shin, Asa, Woodhouse, Smyth and Ezzat 26 , Reference Xie, Hu, Yu, Gu, Wang and Zhang 27
The histological subtype of the PAs was confirmed by immunohistochemical staining. In total, taking the reported 55 cases and adding our 12 cases with histological subtypes, there were 19 NFPAs, 19 prolactinomas, 13 GH adenomas, eight ACTH adenomas, and five mixed adenomas. The other three cases were classified as chromophobe or eosinophilic adenomas. Among them, the data on age, gender, symptoms, and location were available in 36 patients. The characteristics of different types of PA concomitant with RCC are shown in Table 3. Most of the NFPAs associated with RCC presented with mass effect, whereas functional PAs with RCC showed endocrine symptoms. This may indicate that the symptoms of these collision lesions are mainly caused by the PAs. The NFPAs and GH adenomas coexisting with RCC are mainly located at intra- and suprasellar region, whereas ACTH adenomas with RCC are predominately located at intrasellar area. Histological examinations showed that the tumors had two different components. The major component was uniform and polygonal adenoma cells arranged in sheets and nests; the other cystic space was lined by epithelial cells. The cyst wall was typically composed of simple cuboidal or ciliated columnar epithelium and mucous-secreting cells that were pathognomonic for RCC.Reference Miyagi, Iwasaki and Shibuya 8 , Reference Nishio, Fujiwara, Morioka and Fukui 10 Occasionally, the RCCs were partially or completely lined by stratified squamous epithelium or had squamous metaplasia, which is also a characteristic of craniopharyngioma. In such cases, the lack of a solid component and the presence of extensive ciliation and/or mucin production suggested the possibility of RCC.Reference Karavitaki, Cudlip, Adams and Wass 28 The cyst contents were usually a thick, mucoid material consisting of cholesterol and protein, which in surgical series have been described as yellowish (15%-37%), mucoid (51%-70%), or gelatinous (10%).Reference Trifanescu, Ansorge, Wass, Grossman and Karavitaki 29 Immunohistochemical analysis was helpful for classifying PAs and diagnosing RCC. The positive expression of cytokeratin can be used to differentiate RCCs from other lesions.Reference Xin, Rubin and McKeever 30 In our cases, there were two patients with microadenoma of the pituitary and concomitant RCC. The preoperative diagnosis of microadenoma coexisting with RCC was very difficult. Most of the diagnosis was based on histological results; therefore, the histological examinations were very important. After hematoxylin-eosin and immunohistochemical staining, the two patients were diagnosed as PRL adenoma associated with RCC.
Table 3 Features of different types of PA concomitant with RCC
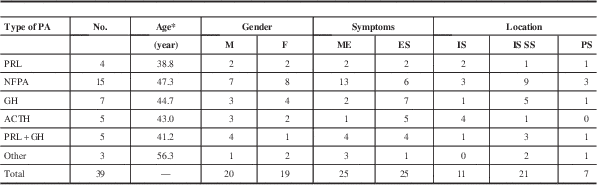
* Average.
ES=endocrine symptom; ME=mass effect; PS=parasellar.
The development of RCC appears simple. The residual lumen of the pouch is reduced to the narrow Rathke’s cleft, which is usually obliterated completely. Failure to obliterate the lumen results in the development of RCC. For PA, similar to other tumors, the pathogenic mechanisms are very complex and still unclear. The development of PAs is thought to involve the interaction of multiple factors, including genetic defects, environmental factors, and endocrine alterations.Reference Rostad 31 , Reference Vandeva, Tichomirowa, Zacharieva, Daly and Beckers 32 The coexistence of PA and RCC has been investigated previously, but the pathological mechanisms remain controversial. There is a notion that Rathke’s pouch proliferates to form the anterior pituitary lobe, from which PAs developReference Trifanescu, Stavrinides and Plaha 33 ; therefore, RCC derived from the remnants of Rathke’s pouch and PAs may have a shared origin. KepesReference Kepes 34 reported the transitional cell tumor of the pituitary gland developing from an RCC. The cells corresponded to an early developmental stage of the pituitary anterior lobe, when the still squamous and columnar Rathke’s cleft cells began to develop their endocrine granulation. Thus, the tumor was derived from “transitional” cells between the lining cells of Rathke’s cleft and the glandular cells of the anterior pituitary; however, this theory was questioned by Ikeda et al,Reference Ikeda, Yoshimoto and Katakura 6 who proved that the cyst within a PA differed from cyst found in the embryonic stage of the pituitary gland. Trokoudes et alReference Trokoudes, Walfish, Holgate, Pritzker, Schwartz and Kovacs 35 reported that the pathogenesis of concurrent prolactinoma and RCC derives from prolactin cell stimulation by an existing RCC, resulting in secondary adenoma formation; however, this theory was rejected by Maria et alReference Koutourousiou, Kontogeorgos, Wesseling, Grotenhuis and Seretis 2 because it contradicts the monoclonal origin of PAs. Furthermore, such a theory cannot be applied to other types of PA combined with an RCC. Karavitaki et alReference Karavitaki, Scheithauer and Watt 7 considered that the coexistence of the lesions may be entirely coincidental. Although these coexisting lesions could be considered purely incidental events, such a coincidence seems to be less plausible based on their common histological origin and the number of cases presented in literature. Most recently, Ikeda et alReference Ikeda and Ohhashi 36 indicated that a ruptured RCC contributed a risk factor of PA, with a high incidence of 34%. The authors also pointed out that six of 203 (3%) patients with PA had an associated RCC in their retrospective study. We speculate that an existing RCC is a risk factor of PA; and the ruptured RCC is more likely associated with PA compared with unruptured RCC. However, the mechanisms underlying PA associated with RCC are very complex and may include inflammatory stimulation, histogenetic factors, endocrine effects, and so on. The pathogenesis of PA associated with RCC remains to be clarified.
In summary, the coexistence of PA and RCC is rare. It is usually difficult to make a diagnosis before surgery. When a nonenhancing cyst-like signal is demonstrated on MRI scans in a patient with PA, the possibility of a coexisting RCC should be considered. Transsphenoidal surgery is the recommended treatment approach for most patients, and a satisfactory result can be achieved. Although PA and RCC have common embryological origins, the pathological mechanisms of their coexistence need to be investigated further.
Acknowledgments
This work was supported by grants from the National Key Technology Research and Development Program of Ministry of Science and Technology of China (2015BAI12B04) and the Basic-clinical Cooperation Project of Capital Medical University (1150170156).
Disclosures
The authors do not have anything to disclose.
Statement of Authorship
WW sorted the data and wrote the paper; GJ and WJ were responsible for data collection and interpretation; GL contributed to the histopathology of the paper; JZ revised the paper critically; and LZ was responsible for conception, design, and final appraisal for publication.