Introduction
The 2016 community survey led by the Epilepsy Innovation Institute (Ei2) ascertained that one of the most impairing aspects of living with epilepsy was the unpredictable nature of seizures. 1 Unfortunately, over one-third of people with epilepsy (PWE) are pharmacoresistant to the many existing antiepileptic drugs, and therefore continue to suffer from uncontrolled seizures. Reference Kwan and Sander2 Because seizures often include impaired awareness and loss of motor control, uncontrolled epilepsy carries a higher risk of injury—even death—compared to the general population, is a constant source of worry for PWE and caregivers (CG), and constitutes a major handicap (e.g., inability to drive and work). Reference Jette and Engel3,Reference Keezer, Sisodiya and Sander4 Accurately and efficiently predicting impending seizures hours to minutes before they occur would provide a life-changing treatment alternative to people with uncontrolled epilepsy. Reference Bou Assi, Nguyen, Rihana and Sawan5
Since electroencephalography (EEG) is known to be the most direct measure of epileptiform activity, it has been the primary method for studying preictal mechanisms. Reference Kuhlmann, Lehnertz, Richardson, Schelter and Zaveri6 Unfortunately, despite decades of research, no single seizure prediction algorithm has yet been approved for use in a clinical setting. Some of the challenges limiting clinical translation of intelligent forecasting algorithms proposed by our group and others are their computational complexity (required to function offline on supercomputers) and their inability to be generalized across all patients. Reference Gagliano, Lesage, Bou Assi, Nguyen and Sawan7–Reference Stacey9 It has been reported that prediction algorithms perform at least better than chance in a certain proportion of patients depending on characteristics such as seizure duration and interval Reference Cook, Karoly and Freestone10 and that incorporating such complementary information can increase seizure prediction performances for these patients. Reference Karoly, Cook and Maturana11,Reference Stirling, Cook, Grayden and Karoly12
An interesting piece of information that could potentially be used to recalibrate seizure prediction algorithms and improve their performances is the presence or not of epileptic prodromes. Unlike epileptic auras (which are sensory or psychic symptoms generated by the onset of the seizure discharge and generally leading within seconds to more disabling ictal symptoms), Reference Berg, Berkovic and Brodie13–Reference Perven and So16 prodromes are warning symptoms of unclear origin often different from the patient’s aura and preceding seizures by several minutes to days. Reference Besag and Vasey17 Common prodromal symptoms reported by PWE include mood disturbances (anxiety, irritability, anger etc.), psychomotor slowing, headaches, and tremor. Reference Besag and Vasey17
While auras have been widely explored (being useful in localizing the epileptogenic focus), Reference Palmini and Gloor18,Reference Perven, Yardi and Bulacio19 few studies have focused on prodromes. Three survey or interview studies have reported varying prevalence of prodromes of 6.2% in 500 PWE, 39% in 100 PWE, and 21.6% from the accounts of 150 CG of pediatric PWE. Reference Patel, Ferastraoaru, Gold, Lipnick, Jehle and Haut20–Reference Schulze-Bonhage, Kurth, Carius, Steinhoff and Mayer22 Other investigators took on a prospective approach to study the predictive ability of prodromes and reported a prediction sensitivity of 41.4% in 5 PWE (12/29 seizures) Reference Maiwald, Blumberg, Timmer and Schulze-Bonhage23 and a six-fold increase in seizure likelihood within the 12 hours following prodromal symptoms in 19 PWE. Reference Haut, Hall, Borkowski, Tennen and Lipton24 None were successful at identifying distinguishing traits of PWE who experience predictive prodromes. Notably, characteristics such as age, sex, seizure frequency, seizure type, and duration of epilepsy have not been found to be statistically different in PWE who experience prodromes versus those who do not. Interestingly, no studies have assessed characteristics of seizure severity such as seizure duration, presence of ictal alterations in awareness and/or motor control, and duration of the postictal recovery period. Reference Pottkämper, Hofmeijer, van Waarde and van Putten25
In this study, we first aim to investigate the prevalence and reliability of warning symptoms (prodromes, auras, and other premonitory motor symptoms) through an online survey completed by both PWE and CG. Responses to clinical and demographic questions (including measures of seizure severity) were statistically compared between PWE who reported prodromes and those who did not. Findings are then discussed in the context of seizure prediction.
Methods
Population and Survey Instrument
PWE and CG were reached through Canadian patient organizations (the Canadian Epilepsy Alliance and “Épilepsie section de Québec”) who invited their members to anonymously complete an online survey. This questionnaire was part of a larger survey assessing seizure detection acceptability, which was available in English and French throughout February and March 2020. Reference Herrera-Fortin, Bou Assi, Gagnon and Nguyen26 The survey included a series of questions collecting demographics (e.g., age and sex) and clinical characteristics (e.g., seizure frequency/timing, seizure/epilepsy duration, presence of ictal involuntary movements or impaired awareness, and duration of postictal symptoms). The following three questions regarding seizure prediction were also asked and CG responded on behalf of the PWE under their care:
“What symptoms, if any, warn you (the person with epilepsy under your care) of an oncoming seizure with impaired awareness or involuntary movements? List all recurrent symptoms.”
“How long before the start of a seizure do these symptoms begin?”
“What percent of the time are these warnings followed by a seizure?”
This study was approved by the University of Montreal Hospital Research Centre ethics committee (18.091).
Statistical Analysis
PWE and CG were divided based on their response to the seizure prediction questions. More precisely, respondents who listed at least one warning symptom were included in the warning group and the rest were included in the no warning group. Both PWE and CG making up the warning group were then subdivided into two groups based on the period of time between the appearance of the warning symptom and the more disabling/visible seizure manifestations (patient-reported seizure) which is subsequently called the patient-reported warning time. Respondents who reported a warning time less than 5 minutes were included in the 5-minute warning subgroup. This group includes those who listed subjective nonmotor symptoms (most likely epileptic auras) and those who listed more objective premonitory motor symptoms (e.g., eye twitching, spasms, shaking, etc.). On the other hand, symptoms occurring more than 5 minutes before the beginning of the seizures were considered prodromes. While there is currently no consensus on the period defining prodromes, the 5-minute cut-off time was chosen for three reasons: (1) to compare results to similar studies Reference Patel, Ferastraoaru, Gold, Lipnick, Jehle and Haut20,Reference Kotwas, McGonigal and Trebuchon27,Reference Petitmengin, Baulac and Navarro28 ; (2) to ensure that auras and early ictal manifestations were not included in the prodrome group due to the ambiguity of patient-reported seizure onset; (3) to coincide with the standard prediction horizon used in seizure forecasting studies. In the context of seizure prediction, the 5-minute period preceding seizures is the suggested minimum time for effective seizure abortion intervention. Reference Kuhlmann, Karoly and Freestone8,Reference Bou Assi, Nguyen, Rihana and Sawan29 Respondents who listed at least one warning symptom without specifying the time were included in analyses regarding the entire warning group but excluded from analyses pertaining to the 5-minute warning and prodrome groups.
Presentation of Responses
The prevalence of prodromes and 5-minute symptoms are presented as the percent of the total included respondents (PWE and CG are separate) who were classified exclusively in each group. Moreover, the distributions of responses are presented as the percent of PWE or CG who are in the specific group and who responded to the question.
Comparison and Correlation Tests
The questionnaire contained both multiple-choice and free-answer questions. Quantitative responses, including age, duration of epilepsy, age at onset of seizures, seizure duration, seizure frequency, postictal symptom duration, and seizure prediction specificity, were compared between different groups (5-minute warnings vs. prodromes, 5-minute warnings vs. no warnings, prodromes vs. no warnings, all warnings vs. no warnings) using Welch’s t-tests to evaluate the possible association between the presence of warnings and demographic/clinical characteristics. Similarly, Chi-square tests were used to compare responses of different groups to categorical questions such as the sex of the PWE, the predominant seizure occurrence time (night vs. day), and the presence of impaired awareness or involuntary muscle activity during seizures.
Finally, the existence of correlations between the seizure prediction questions and quantitative responses were assessed using Pearson’s correlation tests. For both CG and PWE, all of the warning time and the specificity responses from the warning group were tested against the quantitative demographic responses (age, onset age, epilepsy duration, seizure duration, seizure frequency, duration of postictal symptoms) from each individual. For each aforementioned test, the Bonferroni correction was applied due to multiple comparisons.
Results
Study Samples
A total of 389 respondents (221 PWE, 168 CG) submitted answers to survey questions. Of them, 25 PWE and 18 CG were excluded because they either: (1) did not adequately submit answers to the demographic questions or (2) did not report any symptoms but selected time intervals and/or reliability levels for symptoms in the second and third questions of the seizure prediction section. Therefore, 196 PWE and 150 CG were included in the analyses. Based on the 327 respondents who indicated their current residence, all Canadian provinces were represented with 40% and 25% from Quebec and Ontario, respectively. The distribution of the province/territory of residence for both PWE and CG is illustrated in Figure 1.

Figure 1: Province/territory of residence of the patient and caregiver respondents. A total of 327 responded to this question: 185 peoples with epilepsy (PWE) and 142 caregivers (CG).
Prevalence of 5-minute Warnings and Prodromes
When asked to list any symptoms which warn of an oncoming seizure with impaired awareness and/or involuntary movement, 60.71% (119/196) of PWE and 43.3% of CG (65/150) listed at least one warning symptom. Overall, 44.9% of PWE make up the 5-minute warning and 12.24% prodrome groups. Of the 150 CG, 27.3% make up the 5-minute warning group and 12% the prodrome group. The distribution of the warning time question responses for the PWE and CG symptom groups are illustrated in Figure 2 and range between less than 30 seconds and more than 24 hours. While the prevalence of prodromes is similar for both CG and PWE, CG reported less 5-minute warning symptoms.

Figure 2: Distribution of responses to question asking how long before the start of a seizure do warning symptoms begin. Percentages represent the number of responses for each answer among the participants who adequately answered this question (119 PWE, 64 CG). Blue portions correspond to the 5-minute warning group and red portions to the prodrome group. (A): responses given by people with epilepsy (PWE). (B): responses given by caregivers (CG).
Demographics
The average patient age was 37.3 ± 13.7 years for the 196 PWE and 19 ± 13.6 years as reported by the 150 CG. The difference in age between the patients of the two population samples can be attributed to the fact that most CG responded to the survey on behalf of their young child (122/146 indicated being the parent of a PWE) while PWE were predominantly adults. Seventy percent of PWE respondents were female, while 53% of CG cared for male PWE. The sex, mean age, and mean duration of epilepsy for those who experienced 5-minute warnings, those who reported prodromes and those without any warning symptoms are presented in Table 1 (PWE) and Table 2 (CG). The distribution of responses to each question for all groups is detailed in Supplementary Table S1 and Table S2.
Table 1: Summary of distribution of responses per group for PWE. For a full list of all questions and distribution of responses, see Supplementary Table S1.

* Statistically significant difference in postictal symptom duration between prodrome group and 5-minute warning group t(46) = −2.9, p = 0.005.
† Statistically significant difference in postictal symptom duration between prodrome and no warning groups t(53) = 2.8, p = 0.006.
‡ Statistically significant positive correlation between postictal symptom duration and reported warning time r = 0.26, n = 104, p = 0.007.
§ Statistically significant positive correlation between epilepsy duration and warning symptom specificity r = 0.35, n = 105, p < 0.001.
Table 2: Summary of distribution of responses per group for CG. For a full list of all questions and distribution of responses, see Supplementary Table S2.

† Statistically significant difference in postictal symptom duration between prodrome and no warning groups, t(26) = 2.9, p = 0.006.
Warning Symptoms
The average number of symptoms listed per respondent was 1.96 for the CG and 2.08 for the PWE. The free-listed symptoms were then categorized into 25 categories based on recurring terms and on common categories of prodromes reported in the literature.Reference Besag and Vasey 17 Due to the free-answer nature of the question, some responses were either unintelligible or irrelevant to the question. Therefore, these responses could not be classified into the relevant categories and were included as “other” as shown in Figure 3. This category corresponded to 18/248 responses from the PWE and 19/127 from the CG. Overall, the most reported symptoms were dizziness/vertigo (25), tremor/spasm/eye twitching (19) and vision problems among the PWE and staring (13), tremor/spasm (12), and nausea/vomiting (9) among the CG. Seventeen respondents (14 PWE, 3 CG) mentioned having “auras” but did not offer further information on these symptoms. Interestingly, none of the 17 respondents who listed “auras” as a warning symptom corresponded to the prodrome group. This may be an indication that prodromes occurring more than 5 minutes before seizures are more distinguishable than the indescribable “aura” sensation immediately preceding seizures. The most common symptoms for PWE experiencing 5-minute warnings (n = 88) were vision problems (17), dizziness/vertigo/loss of balance (16), and tremor/spasm/eye twitches (14). CG reporting 5-minute warnings (n = 41) identified staring/“spacing out” (11) and tremor/spasms/eye twitches (7) as the most common warnings. In contrast, PWE who reported prodromes (24) experienced mostly dizziness/vertigo/loss of balance (8), cognitive changes (5) and mood changes (5). CG who reported prodromes (19) most commonly listed mood changes (6), tremor/spasms/eye twitches (5) and nausea, dizziness, or cognitive changes (4). The distribution of the listed symptoms by the PWE and CG are illustrated in Figure 3. Interestingly, the most listed prodromes were shared between PWE and CG which include mood changes, cognitive changes, and dizziness and were collectively listed by 75% (18/24) of the PWE and 74% (14/19) of the CG prodrome groups. On the other hand, both groups showed more heterogeneity in their reported 5-minute warnings.

Figure 3: Premonitory symptoms freely listed by (A): people with epilepsy (PWE) and (B):caregivers (CG) of PWE. Categories are listed (left to right) in order of decreasing occurrence. Portions listed in blue and orange correspond to symptoms listed as 5-minute warnings and prodromes respectively. Portions in green represent warning symptoms listed by respondents who did not indicate a warning time.
Relationships between Warning Symptoms and Other Variables
Most Reliable Warning Symptoms
When asked to indicate what percentage of time these warning symptoms are followed by a seizure (prediction specificity), 24 CG and 33 PWE reported warning symptoms leading to seizures 91–100% of the time. These symptoms can be considered as the most reliable predictors and were mainly 5-minute warnings (PWE: 27/33, 81.8%; CG: 19/24, 79.2%), specifically warnings immediately preceding seizures (<30 seconds) (PWE: 13/33, 39.4%; CG: 12/24, 50.0%). The most common of these symptoms identified by CG were “staring/spacing out” (6 CG) and “nausea/butterflies/vomiting” (5 CG). For PWE, the most recurring reliable symptoms were “dizziness/vertigo/loss of balance” (10), “Tremor/spasms/eye twitches” (10), and “fatigue/weakness” (6). The distributions of responses to this question for PWE and CG are included in Tables 1 and 2. Interestingly, 91–100% was the most common response to this question among the warning groups (28% of PWE and 37% of CG). This indicates that most respondents who listed warning symptoms did so with a high level of certitude. The median specificities were 51–75% among the PWE and 76–90% among the CG.
Chi-Square and Welch’s t-test: Significant Differences between Symptom Groups
PWE
Welch’s t-tests were used to compare the quantitative question responses between the four subgroups of respondents against a Bonferroni-adjusted confidence level of 0.0071. No significant difference in age, duration of epilepsy, epilepsy onset age, seizure frequency, seizure duration, and prediction specificity were found between any of the groups. The duration of the period with postictal symptoms was found to be significantly longer in the prodrome group than the 5-minute warning group t(46) = −2.9, p = 0.005 and the no warning group t(53) = 2.8, p = 0.006 with 57% of the prodrome group indicating that their period of postictal confusion/impaired awareness lasts more than 1 hour. The median duration was 30 minutes for the 5-minute warning and no warning groups and more than 1 hour for the prodrome group. The distributions of responses to the postictal symptom duration question for both the PWE and CG groups are illustrated in Figure 4 where the difference is visible. When comparing the groups using the Chi-Square test against a corrected alpha level of 0.0125, no significant association was found between the presence of warning symptoms and any of the four categorical characteristics (sex, seizure time of day, and impaired awareness or involuntary movement during seizures).
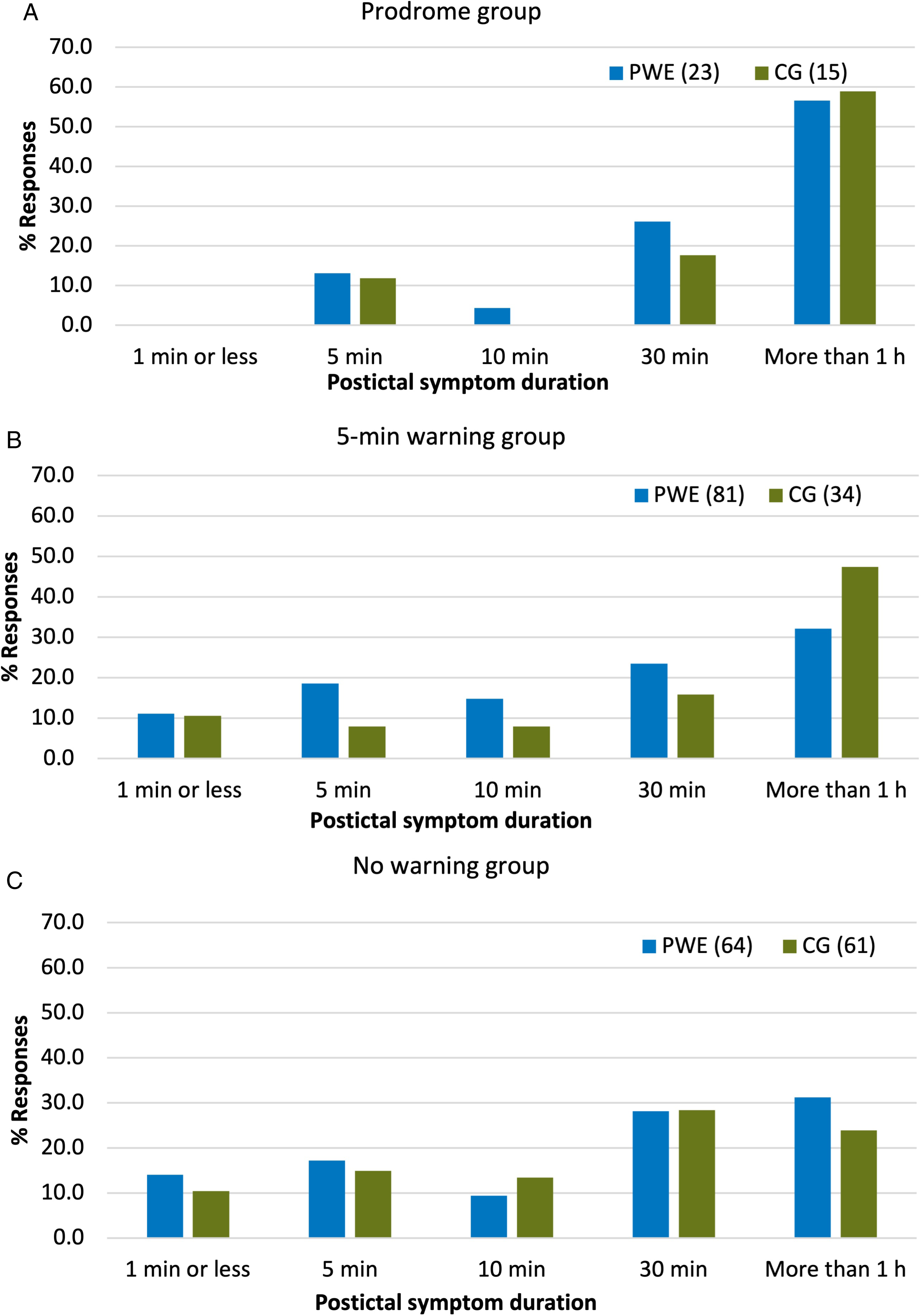
Figure 4: Distribution of responses to duration of patient-reported period of postictal confusion and/or impaired awareness. (A): prodrome group. (B): 5-minute warning group. (C): no warning group. PWE: people with epilepsy. CG: caregivers.
CG
Similarly, the Welch’s t-tests showed no significant difference in age, duration of epilepsy, epilepsy onset age, seizure frequency, seizure duration, and specificity between any of the groups compared against a Bonferroni-adjusted alpha level of 0.0071. However, again, a statistically significantly longer period of postictal symptoms was found in the prodrome group (median = more than 1 hour) compared to the no warning group (median =30 minutes) t(26) = 2.9, p = 0.006. The distributions of the postictal duration responses are included in Table 2. On the contrary, unlike the PWE, significant associations (Bonferroni-adjusted alpha level of 0.0125) were found between the experience of 5-minute warnings versus prodromes and the presence of impaired awareness X 2 (3, N = 59) = 13.93, p = 0.003 as well as involuntary movement during seizures X 2 (2, N = 59) = 26.02, p < 0.001. The presence of these associations only within the CG responses may be attributed to the more objective description of seizure severity that CG provide as well as the possible differences in etiology in the younger population (primarily younger than 18 years) which was represented by the CG. Unfortunately, seizure semiology and epilepsy syndrome were not asked and could therefore not be correlated to the presence of predictive prodromes.
Pearson’s Correlation: Relationships between Quantitative Demographics, Warning Time, and Specificity
The existence of correlations between the warning time, the prediction specificity, and the quantitative characteristics was assessed within the positive symptom groups using Pearson’s correlation at a Bonferroni-adjusted alpha level of 0.008. Firstly, early occurrence of warning symptoms (longer warning time) was found to be positively correlated to longer periods of postictal confusion/impaired awareness in the PWE (r = 0.26, n = 104, p = 0.007). This correlation complements the previously mentioned significantly longer postictal period in the prodrome group. Secondly, the number of years the PWE has been suffering from seizures (duration of epilepsy) was found to be positively correlated with the reported specificity at which they can anticipate their own seizures (r = 0.35, n = 105, p < 0.001). In other words, the more years of experience the patient has with their epilepsy, the higher the percent of time the warning symptom is followed by a seizure.
Discussion
In this study, we have assessed the existence of a distinguishable group of PWE who are able to anticipate their seizures based solely on epileptic prodromes.
Warning Symptom Pevalence
In recent years, while the study of prodromes, based on interviews and EEG analysis, has resurfaced due in part to its potential to improve modern seizure prediction techniques, little work has compared prodromes and 5-minute warnings to better understand and characterize their differences in a large cohort. Here, results showed that 60.71% of PWE and 43.3% of CG can identify at least one symptom which warns them of oncoming seizures and 12% of PWE experience prodromal symptoms occurring at least 5 minutes before the seizure. These results align with the prevalence of prodromes reported in the literature which typically range between 2% and 39% depending on how authors defined prodromes. Reference Besag and Vasey17,Reference Patel, Ferastraoaru, Gold, Lipnick, Jehle and Haut20–Reference Schulze-Bonhage, Kurth, Carius, Steinhoff and Mayer22,Reference Kotwas, McGonigal and Trebuchon27,Reference Alving and Beniczky30,Reference Mackay, Mahlaba, Gavillet and Whittaker31 In this study, the warning times reported ranged between 5 minutes and more than 24 hours with a median response of 2 hours which highlights the importance of subject specific preictal time assessment for seizure prediction.
Prodrome Group and Seizure Severity
The key finding of this study is that the prodrome population can be distinguished from patients whose warning symptoms begin 5-minutes before their seizures or do not experience any warning symptoms by a statistically longer self-reported postictal period of confusion and/or impaired awareness. Postictal manifestations are known to be indications of the severity of the ictal symptoms. Reference Pottkämper, Hofmeijer, van Waarde and van Putten25,Reference Krauss, Edwards, Aminoff and Daroff32,Reference Ohira, Yoshimura, Morimoto, Ariyoshi and Kohara33 In the CG population, the prodrome group also reported having disabling ictal symptoms (impaired awareness and/or involuntary muscle activity) more often than the other groups. Again, seizures with more severe symptoms present prodromal warning symptoms earlier. To our knowledge, this this the first study to correlate the predictability of seizures to the severity of the ictal symptoms. In line with our findings, Cook et al. Reference Cook, Karoly and Freestone10 reported a significant association between seizure duration and greater seizure prediction performances based on intracranial EEG. The concordance between these results suggests that perhaps seizures with greater electrical “intensity” (for which recovery time is longer), manifest as more clinically severe, and may build up earlier within the brain hence being more predictable. Obviously, seizures with more disabling or severe ictal symptoms are the ones that are most important to predict since they present a greater risk for PWE. While no studies comparing prodromal symptoms to EEG analysis have explored this correlation, Petitmengin et al. Reference Petitmengin, Baulac and Navarro28 hypothesized that the occurrence of prodromes between 5 minutes and 24 hours before seizures could result from a preictal loss of connectivity between the epileptogenic focus and its surrounding areas, commonly referred to as functional isolation. Studying the association between the level of preictal functional isolation, measured through intracranial EEG, and the presence of prodromal symptoms could therefore potentially improve the identification of patients whose seizures can be forecasted.
Analysis of Freely Listed Warning Symptoms
The freely listed warning symptoms were very similar between the PWE and the CG which shows that the CG listed both subjective and objective symptoms on behalf of the PWE at their care and suggests that similar phenomena can be observed in the adult and younger population represented by the CG. However, slight differences in the 5-minute warnings may be attributed to the fact that CG are less likely to report subjective experiential symptoms which only patients experience (e.g., vision problems). On the other hand, in both populations, the prodromes listed were noticeably different from the 5-minute warnings which supports the commonly reported hypothesis that seizure prediction is possible in a distinct group of patients. The most common prodromes for both PWE and CG were mood changes, cognitive changes, and dizziness which were collectively listed by 75% and 74% of the PWE and CG prodrome groups. Similarly, according to a recent review article, Reference Besag and Vasey17 among the most common are confusion, anxiety, and irritability. Scaramelli et al. also found behavioral, mood, and cognitive changes to be the most frequently reported prodromes among 100 adult PWE Reference Scaramelli, Braga and Avellanal21 and Cull et al. reported dizziness among the most common. Reference Cull, Fowler and Brown34 In line with our findings, Haut et al. also reported that mood changes (feeling emotional), cognitive changes (concentration difficulty), dizziness, and blurred vision were associated with the most significant increase in seizure occurrence within 12 hours. Reference Haut, Hall, Borkowski, Tennen and Lipton24 These subtle symptoms can be caused by a variety of underlying electrical mechanisms including increased frequency of preictal epileptiform discharges (e.g., spikes, high frequency oscillations, beta, gamma, alpha spindles) in regions inside or connected to the seizure onset zone, brief electrical seizures or brief potentially ictal rhythmic discharges, and variations in connectivity patterns between brain regions (e.g., functional isolation). Interestingly, weakened functional connectivity between brain regions has been shown to be correlated to cognitive symptoms (e.g., impaired memory or concentration) in schizophrenia Reference Erdeniz, Serin, İbadi and Taş35,Reference Lynall, Bassett and Kerwin36 and to good surgical outcomes (Engel I and II post-surgical outcome scores) in PWE who have undergone epilepsy resective surgery. Reference Bou Assi, Zerouali, Robert, Lesage, Pouliot and Nguyen37 Additionally, recent studies have explored cycles in the frequency of epileptiform discharges (subject-specific circadian to multiday cycles) as measured by intracranial EEG Reference Proix, Truccolo and Leguia38 and subscalp EEG Reference Stirling, Karoly and Maturana39 and have reported promising seizure prediction capabilities. Future studies exploring the possible link between variations in the rates of these epileptiform events measured in intracranial EEG recordings and seizure prodromes within the hour to day preceding seizures could greatly improve our understanding of patient-specific preictal mechanisms and guide seizure prediction algorithms. Furthermore, the identification of optimal electrode placement in brain regions expressing patterns of preictal activity would tremendously improve the performance of seizure prediction algorithms as well as their translation to practical real-time devices. Reference Gagliano, Lesage, Bou Assi, Nguyen and Sawan7,Reference Bou Assi, Nguyen, Rihana and Sawan29 Regarding 5-minute warnings, more heterogeneity was found which is expected given that some may be early ictal manifestations with no predictive value. It is also worth noting that overall, 7.25% of symptoms listed by PWE and 15% of those listed by CG were classified as “other” since responses were either uninterpretable, irrelevant, or not medically sound. While a free-answer format was chosen over multiple choice for this question (since respondents are more inclined to answer positively to multiple-choice questions), a considerable portion of the listed symptoms could not be interpreted. In future studies, interview surveys or more concise instructions for describing symptoms could mitigate this limitation.
Prediction Specificity
The specificity at which prodromes can predict oncoming seizures has been reported to be considerably low in the literature. Reference Besag and Vasey17 To address this point, respondents were asked to indicate the percent of the time the listed warning symptoms are followed by a clinical seizure. As expected, respondents who listed 5-minute warnings represented the majority of the respondents who indicated 91–100% specificity. However, among the prodrome group, 29% chose the highest level of specificity. These results highlight the need to characterize patients prodromes and compare them to continuous intracranial EEG monitoring in a large cohort of patients. Reference Alving and Beniczky30 An objective clinical assessment of patients’ prodromal symptoms may improve treatment for a specific group of patients with refractory epilepsy. Reference Scaramelli, Braga and Avellanal21,Reference Kotwas, McGonigal and Trebuchon27 Notably, advances in wearable technology could allow for prodromal symptoms to be collected and incorporated into multimodal seizure forecasting algorithms. Recent studies on multimodal noninvasive seizure prediction have shown adequate prediction performances in machine-learning algorithms when incorporating electronic diary entries Reference Karoly, Cook and Maturana11,Reference Stirling, Cook, Grayden and Karoly12 and prodromes. Reference Cousyn, Navarro and Chavez40,Reference Meisel, El Atrache, Jackson, Schubach, Ufongene and Loddenkemper41 Evidently, PWE who report being able to accurately predict their seizures based solely on prodromes would still greatly benefit from the autonomy that an automatic and reliable seizure prediction algorithm would provide. Moreover, the positive correlation found between the specificity at which PWE reported their prodromes can predict their seizures and the number of years they have been suffering from seizures (P < 0.05) highlights that seizure prediction based on prodromes can be learned through examples. Concordantly, three other studies also reported higher prediction performances in patients who have had epilepsy for a greater number of years, or as we put it, have more experience of their epilepsy. Reference Cull, Fowler and Brown34,Reference DuBois, Boylan, Shiyko, Barr and Deyinsky42,Reference Lee and No43
Limitations
Due to the lack of EEG and information on seizure semiology, the onset of the seizures remains subjective and approximate. For this reason, auras and premonitory motor symptoms warning of oncoming seizures could not be distinguished from early ictal symptoms and predictive prodromes could not be distinguished from mild or brief seizures prior to generalized tonic-clonic seizures. The lack of information regarding different types of seizures also limits the study with regards to the fact that patients who experience retrograde amnesia are less likely to recall seizures with only impaired awareness. This may lead to lower specificity rates or unaccounted prodromes for patients having seizures with impaired awareness. While responses from PWE and CG were kept separate and respondents were asked if they had filled out the survey before to avoid duplicates in the analyses, questionnaires were anonymous and may have been answered by a PWE and their CG. To maximize participation by both PWE and CG, the invitation to participate in the survey was distributed Canada-wide via a post on websites and Facebook pages of Canadian patient organizations. No personalized mail/e-mail invitations were sent; thus, responses were anonymous and the responder rate could not be calculated. This setting did not allow for an independent verification of online data such as clinical and/or phone interviews. Another limitation was that the study is based on retrospective reporting of predictive prodromes. While recall bias (i.e., systemic error caused by respondents being more likely to recall premonitory symptoms after the occurrence of a seizure) could not be avoided in this study, a large cohort of respondents was included and was asked to list general warning symptoms rather than recall individual days. Although similar significant predictive prodromes were reported by Haut et al. in a prospective diary study of 19 patients, Reference Haut, Hall, Borkowski, Tennen and Lipton24 finding should be confirmed in a large cohort of participants in a prospective setting.
Lastly, while the questionnaire was not designed specifically for patients with refractory epilepsy, only 11% of PWE and 5% of CG reported complete seizure freedom. Hence, our patient population is necessarily a good reflection of the overall epilepsy population but is rather biased towards a more pharmacoresistant population. On the other hand, this population is specifically the one that would most benefit from real-time seizure forecasting devices.
Conclusion
Both patient and CG populations support the idea that there exists a distinct group of people for whom seizures are predictable and that this group is different from patients who feel or express warning symptoms within the 5 minutes preceding their seizures. Results show that this population of patients who experience warnings symptoms between 5 minutes and more than 24 hours (median of 2 hours) preceding seizures (prodromes) represents around 12% of PWE and can be distinguished by a significantly longer recovery period of postictal confusion and/or impaired awareness.
The severity of a patient’s seizures may therefore be an indication of the predictability of the seizures. This unexplored avenue of research could lead to breakthroughs in both our understanding of preictal mechanisms and our ability to predict seizures automatically using prodromes and machine learning. Further studies are necessary to validate the ability of prodrome symptoms to predict more severe seizures and to enhance personalized EEG-based seizure prediction algorithms.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2021.499
Acknowledgments
This work was supported by the Canada Research Chair T2 in Epilepsy and Functional anatomy of the human brain [grant number 232075]; the Canadian Institute of Health Research [grant numbers 415079, 453544]; the Institute for Data Valorisation (IVADO) [grant numbers 51628, 51627, 3949094496]; and the Fonds de recherche du Québec – Nature et technologies (FRQNT) [grant number 260530]. The funding sources had no involvement in this study.
Disclosures
Authors have no known or potential conflicts of interest to disclose.
Statement of Authorship
LG, TH-F, and EBA prepared the seizure prediction questions. TH-F and EBA disseminated the questionnaire and collected responses. LG designed and performed the statistical analysis on the collected data and prepared the main manuscript and figures. LG and DKN interpreted the results. FL, MS, and DKN supervised the work. All authors reviewed the manuscript.










