Epidural hematomas (EDH) represent 2.7-4% of traumatic brain injuries (TBI)Reference Bullock, Chesnut and Ghajar 1 - Reference Gupta, Tandon, Mohanty, Asthana and Sharma 3 and have a peak incidence during the second life decade.Reference Bullock, Chesnut and Ghajar 1 , Reference Bricolo and Pasut 4 - Reference van den Brink, Zwienenberg, Zandee, van der Meer, Maas and Avezaat 8 The source of bleeding can be an injured middle meningeal artery, diploic vein or venous sinus.Reference Bullock, Chesnut and Ghajar 1 The Brain Trauma Foundation (BTF) surgical guidelines, although based on class III studies, are widely accepted. They provide objective criteria for deciding either surgical or conservative management in EDH patients.Reference Bullock, Chesnut and Ghajar 1 Yet those who are managed non-operatively remain a concern for the treating neurosurgeon as EDH progression (EDHP) may alter the course of conservative approach. Incidence of EDHP needing surgical intervention is reported to range between 6.25-32% of EDH patients treated initially conservatively in larger studies adopting BTF guidelines or, before its publication, studies that had more stringent criteria for conservative approach.Reference Bezircioğlu, Erşahin, Demirçivi, Yurt, Dönertaş and Tektaş 9 - Reference Sullivan, Jarvik and Cohen 12 One study had 23% EDHP in the sample but only 10% required evacuation.Reference Sullivan, Jarvik and Cohen 12 Another study had 32% of its conservative group (59 patients) complicated with EDHP and 22% of them required surgical intervention.Reference Mayr, Troyer and Kastenberger 10 The other authors had to operate on a single EDHP case each.Reference Bezircioğlu, Erşahin, Demirçivi, Yurt, Dönertaş and Tektaş 9 , Reference Salama and Eissa 11 Epidural hematoma progression represented 7.9% of all cases of TBI progression in a randomized controlled trial.Reference Ding, Yuan and Guo 13 The risk factors of such progression are still not very obvious and the literature has seldom addressed this issue with specific attention and mostly in studies prior to the publication of the BTF surgical guidelines. We carried out this study to identify which patients, among those candidates with EDH, suitable for an initial non-operative treatment, are at greater risk of EDHP, and to determine when they become surgical candidates.
Methods
Patient population
The current study is a Case-Control study in which we retrospectively reviewed the charts of TBI patients who were admitted, over a five-year period, to the Montreal General Hospital, a level 1 trauma center, and one of only three adult level 1 trauma centers serving the province of Quebec, Canada, which has a population of almost eight million people. Nearly 2800 patients were admitted with a diagnosis of TBI during that five-year period. The Montreal General Hospital Traumatic Brain Injury Database and the Trauma Registry Database were used to identify all patients admitted between January 1st, 2006 and December 31st, 2010 with a diagnosis of traumatic EDH. Our inclusion criteria were: all patients with a traumatic EDH, with or without other brain injury types, for whom conservative management approach was planned initially. Those who had cranial surgery for a lesion other than EDH on the opposite side were included. Patients who were admitted for urgent surgery, those who were deemed non salvageable at presentation, or those for whom the initial (CT) images were acquired at another institution and were not available for review were excluded. Patients for whom charts were incomplete were also excluded.
Patient management
All patients with a traumatic EDH were evaluated by a dedicated trauma team and by the neurosurgery service. Patients requiring immediate surgery were directed to the operating room. The study spans a time period shortly before the publication and application of the BTF surgical guidelines. Therefore the decision to operate immediately, versus conservative management, was left to the treating neurosurgeon. Patients with small EDH (<30 cm3 ) and no associated midline shift or deficit were initially conservatively treated. They were observed in the emergency department or in the intensive care unit under close monitoring for at least 24 hours. The CT scans were routinely done upon presentation then routinely repeated within 6–12 hours and whenever neurological deterioration occurred. Patients who were admitted to the intensive care unit underwent a second routine follow-up CT scan after 48 hours if venous thrombo-prophylaxis was to be initiated.
Data collection
The charts were reviewed for demographic data, TBI severity using Glasgow Coma Scale (GCS), global injury severity using the Injury Severity Score (ISS), method of injury, neurological status at presentation, use of anticoagulants or anti-platelet medications, presence of alcohol intoxication or coagulopathy, defined as an International Randomized ratio (INR) >1.2, or a partial thromboplastin time (PTT) >50 seconds for the first 24 hours following admission. The CT scans were reviewed to retrieve EDH site and parameters, note any mass effect or other cranial injuries and measure the midline shift (MLS). The size of the lesions was collected in three dimensions. The width was measured as the transverse diameter, the length as antero-posterior diameter and the depth as the supero-inferior diameter. For use in the regressions, we computed an approximated volume by multiplying the three dimensions using the equation: volume=ABC/2.Reference Petersen and Espersen 14 The time delay between the initial CT and the follow up CT was also recorded
Outcome measures
The primary outcome measures were: (1) the percentage of patients initially treated conservatively who required eventual surgical evacuation of their EDH; (2) the timing for that delayed surgery; (3) the reason for requiring this delayed surgical evacuation; (4) the method used for surgical evacuation. Secondary outcome measure was the extended Glasgow Outcome Scale (GOSE) scoreReference Jennett and Bond 15 - Reference Jennett, Snoek and Bond 16 at discharge from the acute care hospital, whether the discharge destination was home or another medical facility. The GOSE was always assigned according to a consensus within the multidisciplinary team at discharge from the acute care hospital.
Statistics
The patients were categorized into two groups: “observation” group with successful conservative approach and “EDHP” group that required urgent surgical evacuation. After statistically comparing both groups’ characteristics, Bivariate Association statistical analysis was used to associate risk factors with EDHP. A logistic regression was run to predict the occurrence of EDPH and subsequent surgery. Subsequently, a stepwise logistic regression (pin<0.05) was used and the significantly different variables were entered in the model. Outcomes were assessed by linear and bivariate regressions using the GOSE scale.
Results
The need for surgical evacuation
A total of 201 patients with a diagnosis of EDH were retrieved from our center's TBI registry. Of those, 30 were excluded because they had been wrongly labelled as EDH, and five were excluded because only comfort measures were offered (two patients), or the data were incomplete (three patients). Therefore, 166 patients had a diagnosis of traumatic EDH and were actively treated. Of those, 41 required emergency evacuation (24.7%) and 125 patients (75.3%) were initially observed and were therefore included in the present study. The majority (111 patients or 88.8%) of the patients with EDH who were initially observed remained non-operative, and only 14 patients (11.2%) required delayed surgery. Therefore, of all the patients with traumatic EDH actively treated, 55 eventually required surgical evacuation of the EDH (33.1%). Figure 1 illustrates the organisation of the sample.

Figure 1 Distribution of all treated epidural hematoma between immediate surgical treatment, initial conservative treatment and failed conservative treatment.
Demographic data (age, GCS, ISS, mechanism of injury, alcohol intoxication)
The mean age in the entire sample was 39.1+/−18.0 years of age (range 16-96). Figure 2 depicts the age distribution of the sample. Three patients had bilateral EDH. In order to avoid having dependence in the sample, only one lesion was chosen randomly in those patients. Men composed the majority of the sample (81.6%). Glasgow Coma Score at admission varied between 3 and 15 with a mean (±SD) of 12.6±3.4 and a median of 14. The majority of the sample (62.4%) had a mild TBI (GCS after resuscitation between 13 and 15), and 14.4 % had a severe TBI (GCS 3-8). The average (±SD) ISS score was 27.5±8.6. Sixty-one percent of the sample experienced a loss of consciousness at the time of the trauma. Among those who did not experience loss of consciousness, 23.4% experienced amnesia. The most frequent mechanisms of injury were fall from height (27.9%) and assault (20.5%). Table 1 gives the relative incidence of each mechanism of injury. Of the 115 patients who have been tested, 40.9% tested positive for blood ethanol.
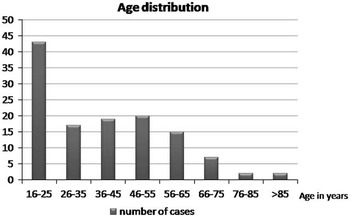
Figure 2 Distribution of age in the sample.
Table 1 Frequency of mechanism of trauma (n=122)
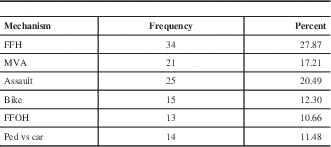
FFH: Fall from height, MVA: Motor vehicle accident, FFOH: Fall from own height, ped vs car: pedestrian versus car
Coagulation
Of the 108 patients who had the information in their chart, 3.7% were using anti-platelets or anticoagulants but none of them was among the EDHP group. Of the 114 patients whose coagulation profiles were tested, 19.3% had coagulation abnormalities.
EDH site and size
A little more than half (56.4%) had a right sided lesion. Almost half of the lesions were temporal (48.0%) (see Table 2 for location distribution). The mean (±SD) width, length, depth and volume were 10.2 (±11.4) mm, 30.7 (±15.5) mm, 26.2 (±18.2) mm and 9.788.7 (±15.390.1) cm3 respectively. More than 93% of the sample had less than 2mm MLS. A large majority of the patients (72.0%) had related fractures but only 5.6% were depressed fractures.
Table 2 Epidural hematoma location distribution (n=125)

Post. fossa= posterior fossa
Detection of EDHP
All of the EDHP patients needed evacuation. Five patients had EDHP detected because they showed signs of neurological deterioration and a CT was repeated urgently. However, EDHP was detected by routine follow up CTs in most of those with mild TBI (six out of eight patients) and half of those with moderate (two of four) and severe (one of two) TBI. The initial volume of EDH that eventually progressed was 13 cm3 on average, while the volume at progression was 21 cm3. Time delay for EDHP occurrence ranged between 5–30 hours (h) from the initial CT, with a mean time of 12.4h. Two patients had a 30h time interval for the EDHP to progress. One of those two actually sought medical attention only two days after the trauma. Hence the progression of EDH in that case was much delayed.
Bivariate associations between patient and trauma characteristics and surgery event
The EDHP group who had surgery was significantly younger than the other group (p<0.0001). Among the non-operated patients, 16 out of the 101 tested for coagulation had abnormalities detected (16.7%). This was significantly higher in the EDHP group where 6 of the 13 tested patients (46.1%) had documented coagulation abnormality (p=0.009).
There were no differences in gender, ISS scores, GCS scores, proportion of loss of consciousness, mechanism of trauma, proportion of presence of alcohol in the blood, proportion of use of anticoagulant or anti-platelet therapy, side of the lesion, location of the lesion, midline shift, proportion of associated fractures, proportion of depressed fractures, any of the dimensions of size or volume of the lesion between the EDHP and non-surgical groups. Table 3 lists all the variables, the statistical test used to compare them and the p values for each.
Table 3 Statistical comparison of surgery v. no surgery groups
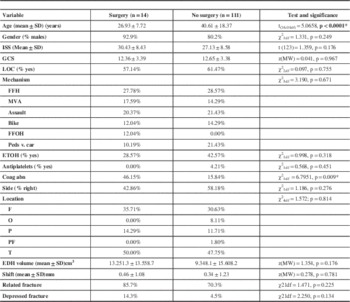
Coag abn= coagulation abnormality, FFH= fall from height, FFOH= Fall from own height, GCS= Glasgow Coma Scale, ISS= Injury severity scale, LOC: Loss of consciousness, MVA= Motor vehicle accident, ped vs car= pedestrian versus car, F= frontal, O= occipital, P= Parietal, PF= parietofrontal, T=Temporal. ETOH=alcohol abuse, EDH= epidural hematoma, mm= millimeters, SD= standard deviation, *Statistically significant.
Prediction model for EDHP
Age was entered as a control variable since the groups had different mean ages. Increasing age decreases the odds of having surgery (odds ratio (OR) =0.94; 95% confidence interval (CI) =[0.91; 0.97]). After controlling for age, having a coagulation abnormality increased the odds of having surgery by an average of 6 times (OR=6.12, 95% CI=[1.54; 24.36]) but the volume of the lesion was an insignificant predictor. Table 4 shows the exponentiated coefficients (OR) for each variable in the model as well as their significance.
Table 4 Results of the logistic regression predicting the event of surgery
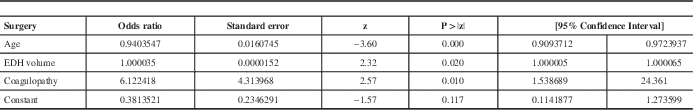
EDH: Epidural hematoma
Figure 3 shows the ROC curve of this regression model. The area under the curve is 0.813 and is significantly larger than 0.5, indicating those variables are important predictors of the occurrence of surgery. A sensitivity/specificity plot as in Figure 4 shows that when the probability of surgery is larger than 12.5% using the three variables in the logistic regression, the sensitivity of the predictive model is 77% and the specificity is 80%.

Figure 3 Receiver operating characteristic (ROC) curve of the prediction of surgery using the three variables (age, abnormal coagulation and hematoma volume) model. The total number of patient is 113 as coagulation as all three variables were known in 113 patients.

Figure 4 Sensitivity and specificity plot according to the probability of surgery.
Outcome
Seven patients (5.6%) died and a small proportion of subjects had severe disability (7.2%). The majority of the sample (87.2%) had a good recovery (GOSE between 5 and 7) early in evolution. Table 5 gives the distribution of the GOSE scores.
Table 5 Frequency of GOS scores (n=125)

GOS= Glasgow Outcome Scale score
Having progression of the EDH was not associated (χ2 KW1df=0.318, p=0.5730) with a better or worse outcome (GOSE score). Even when controlling for age, coagulation abnormality and volume of the lesion, having EDHP was not a significant predictor of the outcome in an ordinal logistic regression (OR=0.644, p=0.604).
Discussion
This study has demonstrated that, contrary to common belief, the majority of traumatic EDH are not surgical emergencies with 75% of the cases initially treated conservatively. Furthermore, even when accounting for EDHP, two thirds of the EDH patients never required surgery. The main factors leading to EDHP found in this study were a younger age and coagulation abnormalities during the first 24 hours after presentation.
Risk factors for EDHP
The causes of EDHP have not been well studied. A recent randomized control trial (RCT)Reference Ding, Yuan and Guo 13 examined the role of routine serial CT scans in TBI and reported that 79 patients (46.2% of the sample) had progression of TBI, but only ten of these 79 had EDH (7.9%). Their sample also included delayed onset of hematomas. The latter should be better considered a different categoryReference Sullivan, Jarvik and Cohen 12 , Reference Domenicucci, Signorini, Strzelecki and Delfini 17 and is outside the spectrum of our study. Risk factors for all hematomas progression were: higher D-dimer concentration, lower GCS on presentation, higher INR and to a lesser extent, shorter time lapse between trauma onset and initial CT. The finding of abnormality in coagulation profile correlates with our study. Bhau et alReference Bhau, Bhau, Dhar, Kachroo, Babu and Chrungoo 18 quoted a rate of 25% of patients who failed conservative treatment out of 89 patients but also did not differentiate EDHP from delayed onset EDH and reported no statistical analysis of potential risk factors for progression. A prospective seriesReference Bezircioğlu, Erşahin, Demirçivi, Yurt, Dönertaş and Tektaş 9 of 80 EDH patients of volume <30 ml treated conservatively concluded that in the five patients (6.25%) who developed EDHP, the only significant association was temporal location. This could be overestimated because of the smaller number and exclusively temporally located cases of EDHP. Sullivan et al,Reference Sullivan, Jarvik and Cohen 12 in a retrospective study of 160 patients treated conservatively, found only higher revised trauma score (implying mild multisystem injury) to be significantly correlated to EDHP. In our study, ISS had no correlation to EDHP. Another small retrospective studyReference Knuckey, Gelbard and Epstein 19 reported 7 of 22 patients developed EDHP. Initial CT<6 hours from onset of trauma and skull fractures traversing major vascular structures were significant risk factors in that study. Injury to first CT time lapse was not investigated in our study. The significant correlation with younger age in our study is a new finding, yet it is not surprising. Indeed, it could be explained by the fact that, in older people, dura matter is more adherent to the internal table of the skull, leaving a faster tamponade effect and less potential epidural space in which the EDH may accumulate. Why it was not picked up in previous studies is not clear but one possible reason might be that our study includes patients of all ages, with a wide range of distribution and not only younger people. Finally, the volume of the hematoma was not a predictor of progression in our study, nor was it in any of the other studies. This is likely due to the fact that all hematomas treated conservatively were of small size.
Timing of follow up CT scans
The literature is not in favor of routine follow up CTs in mild TBI, as a recent meta-analysisReference Almenawer, Bogza and Yarascavitch 20 revealed, but does recommend it for moderate and severe TBI. The question of optimal timing for follow up CTs remains, however, unanswered.Reference Ding, Yuan and Guo 13 , Reference AbdelFattah, Eastman and Aldy 21 - Reference Sifri, Homnick and Vaynman 23 In their RCT, Ding et al.Reference Ding, Yuan and Guo 13 performed serial follow up CTs, scheduled as follows: 6-8h, 20-22h, 48h and seven days for one TBI group and only when neurologically indicated for the controls. This resulted in significantly shorter stay in intensive care and total hospital stay, better GCS at discharge, and less spending among patients who developed progression of injury in the intervention arm. They recommended restricting routine CTs to patients with moderate or severe TBI. Another prospective cohort of mild TBI reported that hospital stay is reduced if CT scans were done only when clinically dictated.Reference AbdelFattah, Eastman and Aldy 21 Figget et al.Reference Figg, Burry and Vander Kolk 24 found, in a retrospective series, that repeat CT scan after 24-48 h doesn't change the management of severe nonsurgical TBI. Our study shows that most EDH patients treated conservatively according to the BTF guidelines had mild TBI. Most of our EDHP cases with initial mild TBI were diagnosed for progression on planned follow up scans. This may suggest that EDH should be considered a different subcategory of mild TBI in terms of necessity for routine serial CT scans follow up.
Time interval for EDHP
Progression of initially non-surgical EDH mostly occurs within the first 24h, less likely within 48h and rarely beyond that. However, it did not occur before at least five hours. As identifying the actual injury time cannot be verified in many trauma cases, we supposed that the time elapsed from the initial CT was more practical. The Ding et alReference Ding, Yuan and Guo 13 RCT reported that 80% of patients (56 out of 70) complicated with progression of TBI did so within 24h and the remaining 20% were delayed 24-48 h. Epidural hematoma, non-operatively treated in accordance with the guidelines, in a prospective non-controlled study,Reference Salama and Eissa 11 have shown EDHP in 10% of patients, occurring within 12h in 6 out of 70 patients and 24 h in 1 patient. However, they excluded another patient who developed EDHP after four days. In their larger study, Sullivan et al.Reference Sullivan, Jarvik and Cohen 12 had all EDHP diagnosed within 36 h interval from trauma onset and mean of 5.3 h from the initial CT scan. The smaller study of Knuckey et al.Reference Knuckey, Gelbard and Epstein 19 mentioned a mean time for EDHP of 2.7 days after admission (range of 1-10 days) in 7 of 22 patients. The critical period in their opinion was the first 24h (71% of EDHP in their sample). Our EDHP events ranged from 5 to 30 h (mean 13.85h) after the initial CT.
Coagulopathy and EDHP
Coagulopathy has been shown to cause progression of TBI. Recently, a retrospective studyReference Mayr, Troyer and Kastenberger 10 investigated the impact of coagulopathy on EDH outcomes of 85 patients, triaged in compliance with the BTF guidelines, into surgical and conservative groups. It showed a significant negative influence of coagulopathy on the outcomes of both groups but found no correlation with EDHP. This result is questionable when considering the smaller sample number and lack of multivariate analysis. There are variable data in the literature and different studies associated one or more of the following with TBI progression: Prothrombin time (PT) or INR, Partial thromboplastin time (PTT), thrombocytopenia, high fibrin degradation and low fibrinogen levels.Reference Allard, Scarpelini and Rhind 25 - Reference Stein, Young, Talucci, Greenbaum and Ross 30 Fewer other studies reported no association between coagulopathy and bleeding progression in TBI.Reference Chang, Meeker and Holland 31 - Reference Sawada, Sadamitsu, Sakamoto, Ikemura, Yoshioka and Sugimoto 33 This considerable variation is attributed to lack of consensus on TBI-coagulopathy definition, heterogeneity of patients involved, variable laboratory tests used in different studies and timing to perform these tests and CTs.Reference Laroche, Kutcher, Huang, Cohen and Manley 34 - Reference Zhang, Jiang, Liu, Watkins, Zhang and Dong 35 The presence of hypodense areas within the epidural hematoma on CT scan is thought to be related to coagulopathy,Reference Hamilton and Wallace 36 yet its association with EDHP is lacking adequate investigations.
TBI associated coagulopathy
Nearly half of our EDHP patients had coagulopathy, despite the fact that none of the patients (one with missing data) were on anti-platelet or anticoagulation medication. This raises the suspicion of TBI-associated coagulopathy, which has an uncertain pathophysiology.Reference Laroche, Kutcher, Huang, Cohen and Manley 34 - Reference Zhang, Jiang, Liu, Watkins, Zhang and Dong 35 The overall incidence of TBI-associated coagulopathy in a meta-analysis was 32.7% and was significantly associated with mortality and unfavorable outcome.Reference Harhangi, Kompanje, Leebeek and Maas 37 A multicenter prospective study reported that within 6h from injury, 36% of its TBI sample fulfilled the criteria for overt disseminated intravascular coagulopathy (DIC) which proved to have a significant correlation with hemorrhage progression. All other causes of coagulopathy were excluded in that study.Reference Sun, Wang and Wu 38 A more recent multicenter studyReference Franschman, Boer and Andriessen 39 demonstrated that both delayed and early sustained coagulopathy in isolated TBI correlates with more abnormalities on initial CT, hematomas >25 ml and worse outcomes in comparison with early short-term coagulopathy.
Outcome
The cause of EDHP is supposedly a re-hemorrhage event or continuous slow bleeding.Reference Sullivan, Jarvik and Cohen 12 , Reference Bhau, Bhau, Dhar, Kachroo, Babu and Chrungoo 18 Brain Trauma Foundation guidelines for successful EDH conservative management states that: “An EDH less than 30 cm3 and with less than a 15-mm thickness and with less than a 5-mm midline shift (MLS) in patients with a GCS score greater than 8 without focal deficit can be managed non-operatively with serial computed tomography (CT) scanning and close neurological observation in a neurosurgical center”.Reference van den Brink, Zwienenberg, Zandee, van der Meer, Maas and Avezaat 8 The safety of these guidelines for conservative treatment of EDH was recently tested in a prospective non-controlled study of 70 patients which showed its safety but emphasized the need for close observation and serial CT scans.Reference Salama and Eissa 11 Brain Trauma Foundation guidelines proved to be safe and provided good outcomes for EDH patients treated conservatively. Should EDHP develop, then timely surgical intervention can maintain similar outcomes to the successful conservatively treated counterparts, as this study and all other related studies have shown.Reference Mayr, Troyer and Kastenberger 10 - Reference Sullivan, Jarvik and Cohen 12
The early outcome of our cohort was favorable for the great majority. A small number died or had severe disability. A poor outcome was not linked to EDH progression however, but rather to the patients’ initial injury severity and older age.
Limitations of our study include its retrospective nature and a small number of patients with missing data. On the other hand, the research question was very specific and the sample number was adequate to perform statistical comparison and multivariate analysis. Another limitation of our study is that the outcome was measured systematically for all patients early in the evolution of the patients (at discharge from acute care hospital). While most of the patients already fell into the category of “good outcome”, the long term outcome would likely be better still.
Furthermore, all patients with EDH were included in this study, and patients with concomitant injuries were included as well. The patients’ outcome could therefore be influenced by the severity of the initial injury and not only by the EDH. The advantage of including patients with concomitant injuries in this study is that the results can be extrapolated to all patients with EDH and not only those with pure EDH, as the latter category does not in fact represent the majority of EDH seen in clinical practice.
Conclusion
Traumatic EDH can be successfully treated conservatively in the majority of cases. A small proportion of these non-surgical EDH will progress and require surgical evacuation. Increased vigilance is indicated for younger adults and those with coagulopathy. Routine follow up CT scans should be done, but the best time frame remains unclear. However, early detection of EDHP and urgent evacuation results in similar outcomes to patients with fully successful conservative management.
Disclosures
None of the authors have anything to disclose.
None of the authors have any competing financial interests.













