Introduction
The superior parietal lobule (SPL) is separated from the inferior parietal lobule (IPL) by the intraparietal sulcus. It is separated anteriorly from the postcentral gyrus by the postcentral sulcus. It continues onto the medial surface of the ipsilateral hemisphere as the precuneus.
The SPL plays a strategic role in many sensory and cognitive processes, including somatosensory and visuomotor integration, Reference Culham, Cavina-Pratesi and Singhal1,Reference Iacoboni2 motor learning, Reference Weiss, Marshall, Zilles and Fink3,Reference Wenderoth, Debaere, Sunaert, van Hecke and Swinnen4 spatial perception, Reference Weiss, Marshall, Zilles and Fink3 mental rotation, Reference Vingerhoets, de Lange, Vandemaele, Deblaere and Achten5,Reference Wolbers, Weiller and Buchel6 visuospatial attention, Reference Corbetta, Shulman, Miezin and Petersen7 and memory. Reference Lacquaniti, Perani and Guigon8,Reference Zago and Tzourio-Mazoyer9 The SPL was first defined in the Brodmann atlas as including two anteriorly to posteriorly arranged subareas, BA 5 and BA 7. Reference Brodmann10 The anterior BA5 has been described as primarily integrating information from the somatosensory cortex and providing the dorsal premotor cortex (dPM) with a spatial representation of the body parts. Reference Jones, Coulter and Hendry11,Reference Mountcastle, Lynch, Georgopoulos, Sakata and Acuna12,Reference Pandya and Seltzer13 The posterior BA7, in contrast, was considered to receive input from the visual cortex and provide visual information for the dPM in the context of visually guided reaching movements. Reference Battaglia-Mayer and Caminiti14,Reference Caminiti, Ferraina and Johnson15,16 Moreover, the SPL (areas 5,7) has reciprocal connections with Sm I and with the dorsal tier of lateral thalamic nuclei. SPL is concerned with discriminative aspects of sensation, such as the qualities of shape, roughness, size, and texture, and also in remembering the positions of objects in space. It is responsible for a general awareness of the contralateral body image and the body part localization processing.
Consistent with the established role of the parietal cortex in multisensory integration, many sensory and cognitive process disorders have been reported following damage of the SPL, most frequently in the right hemisphere. Reference Vuilleumier, Reverdin and Landis17,Reference Daprati, Sirigu and Nico18 Moreover, the anterior subregions of SPL were involved in action processes and in visually guided visuomotor functions, and the posterior subregions were associated with visual perception, spatial cognition, reasoning, working memory, and attention. Reference Wang, Yang and Fan19 In connection with these data, many neuropsychological studies have reported associations between SPL (with adjacent precuneus) and different manifestations of somatosensory and visual motor integration disorders, in particular xenomelia, anosodiaphoria, Doppelgänger, visuospatial neglect, optic ataxia, and optic apraxia; however, the precise contribution of SPL to these abnormal findings has yet to be determined. In this study, we evaluated a series of consecutive patients to investigate the clinical, neurocognitive, and behavioral features of isolated SPL strokes.
Subjects and Methods
Between January 2013 and October 2019, fourteen patients with ischemic lesions restricted to the SPL were recruited from 4200 patients with ischemic stroke. They were with first-ever stroke consecutively enrolled in the prospective Ege Stroke Registry. A written informed consent was taken from all patients, and the study was reviewed and approved by the ethics committee of Ege University Medical Center. Our registry is hospital-based, that collects a wide range of clinical and neuropsychological data on hospitalized patients with stroke to determine the risk and cause of cerebrovascular disease and to study cognitive features of cerebrovascular events. Reference Kumral, Ozkaya, Sagduyu, Sirin, Vardarli and Pehlivan20 All patients had a standardized evaluation for their clinical and neuropsychological pictures, and consecutively recorded variables included age, gender, stroke risk factors, blood pressure, etiological subtypes, topography of lesions on CT and MRI. CT and magnetic resonance, 2D-echocardiography, and 24-hour electrocardiography (Holter) monitoring were performed in all cases. The cause of stroke was assessed according to the criteria described previously as large-artery disease, small-artery disease, cardioembolic, others, and unknown. Reference Adams, Bendixen and Kappelle21 Patients with previous stroke, old lesions on neuroimaging, or concomitant acute multiple lesions outside the SPL were excluded.
Discriminative aspects of sensation, such as the qualities of shape, roughness, size and texture, and remembering the positions of objects in space, were assessed on the first day of stroke. All patients were examined for a general awareness of the contralateral body image and the location of its parts. They were asked to identify familiar objects manually and examined for awareness of the side of the body opposite to the lesion. Based on the body awareness disorders, several aspects of body schema and body image disorders (BSIDs) were systematically assessed in all patients. This included alien hand syndrome, tactile allochiria, anosognosia for hemiplegia, autotopagnosia for body parts or for body sensations, motor neglect, fading limb, finger agnosia, micro- or macrosomatognosia, supernumerary limb, hemiconcern, xenomelia, anosodiaphoria, and tactile extinction. Definition and assessment of BSIDs are presented in Table 1.
Table 1: Definition of clinical terms and syndromes

Assessment of Other Neurocognitive Impairments
Based on the current knowledge about the SPL, different aspects of neuropsychological impairments were systematically assessed by a test battery. The neurocognitive assessment and the BSIDs were conducted by two neuropsychologists (H.A., G.Ç.) with accreditation in neurocognitive science in the first week of stroke. A z-score of each patients’ neurocognitive test point was calculated, and a score below the cutoff or a z-score inferior to -2 was considered as pathological. Topographical memory was examined by Topographical Recognition Memory Test (to find the difficulties in perceiving topographical features or the retention of topographical information over very short intervals) (normal mean+SD, 30 + 6); episodic memory by Rey Auditory Verbal Learning Test, immediate free recall/new learning subtest (List A Trials 1–3) (normal mean+SD, 6.3 + 2.1); semantic memory by Controlled Oral Word Association Test (normal mean+SD of animals named, 18 + 4.5); spatial/topographic orientation by Benton’s Judgment of Line Orientation Test (mean+SD, 11.5 + 2.1); visuospatial neglect by the bells test (normal mean+SD, 35 + 6); visual extinction by finger movements for 5 times on both visual hemi-fields (normal mean+SD, 5 + 1); motor neglect by observation of underutilization of one side, without defects of strength, reflexes. Other neurological/neuropsychological syndromes were systematically checked such as visual agnosia (tested by “incomplete letters” of the Visual Object and Space Perception Battery) (mean+SD, 20 + 4); constructional apraxia, ideational apraxia, dressing apraxia, optic ataxia (disturbance to reach and grasp the examiner’s finger placed successively in different sectors of the visual field), optic apraxia (disorder of controlled, voluntary, and purposeful eye movement), and left/right indistinction (inability to distinguish between right and left). Language skills such as verbal fluency, comprehension, naming and word finding, repetition, reading, writing, and calculation were tested with the Ege Aphasia Test.
Magnetic Resonance Imaging Acquisition and Data Acquisition, Normalization
MRI was performed within one week after stroke by 3T scanner (Siemens Sonata, Siemens Medical Solutions, Erlangen, Germany) as part of the standard examination. Routine MRI sequences are obtained, including T1-weighted imaging, T2-weighted imaging, DWI, and fluid-attenuated inversion recovery. Two vascular neurologists with accreditation in neuroimaging retrospectively reviewed the noncontrast MRI sequences blinded to clinical variables. The SPL is separated from the IPL by the lower margins of the intraparietal sulcus. Anteriorly, it is separated from the postcentral gyrus by the postcentral sulcus. It continues as the precuneus on the medial surface of the ipsilateral hemisphere. However, patients with precuneal involvement were not included in the study. However, 5 patients had partial involvement of the IPL which is located posterior to the postcentral gyrus and is the inferior component of the parietal lobe. For each normalized individual imaging, we used SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/) with standard parameters applied in the MATLAB environment (2019 version, MathWorks). During this step, all normalized MRIs (MNI152 brain) were manually controlled to verify that all normalizations were of adequate quality. Then, we manually drew the ischemic areas and converted them into binary images using MRIcroGL software (http://www.mccauslandcenter.sc.edu/ mricrogl/home). To assess stroke volume in distinct cognitive groups, we used the nonparametric Mann-Whitney test.
Results
The patient population consisted of seven females and males each, with a mean + standard deviation (SD) age of 65.57 + 6.02 years and a mean educational level of 10.36 + 3.48 years (5-16). Twelve patients were right-handed, and for 8 of them, the ischemic lesion was located in the right hemisphere (Figure 1A). Demographic and clinical features are given in Table 2.
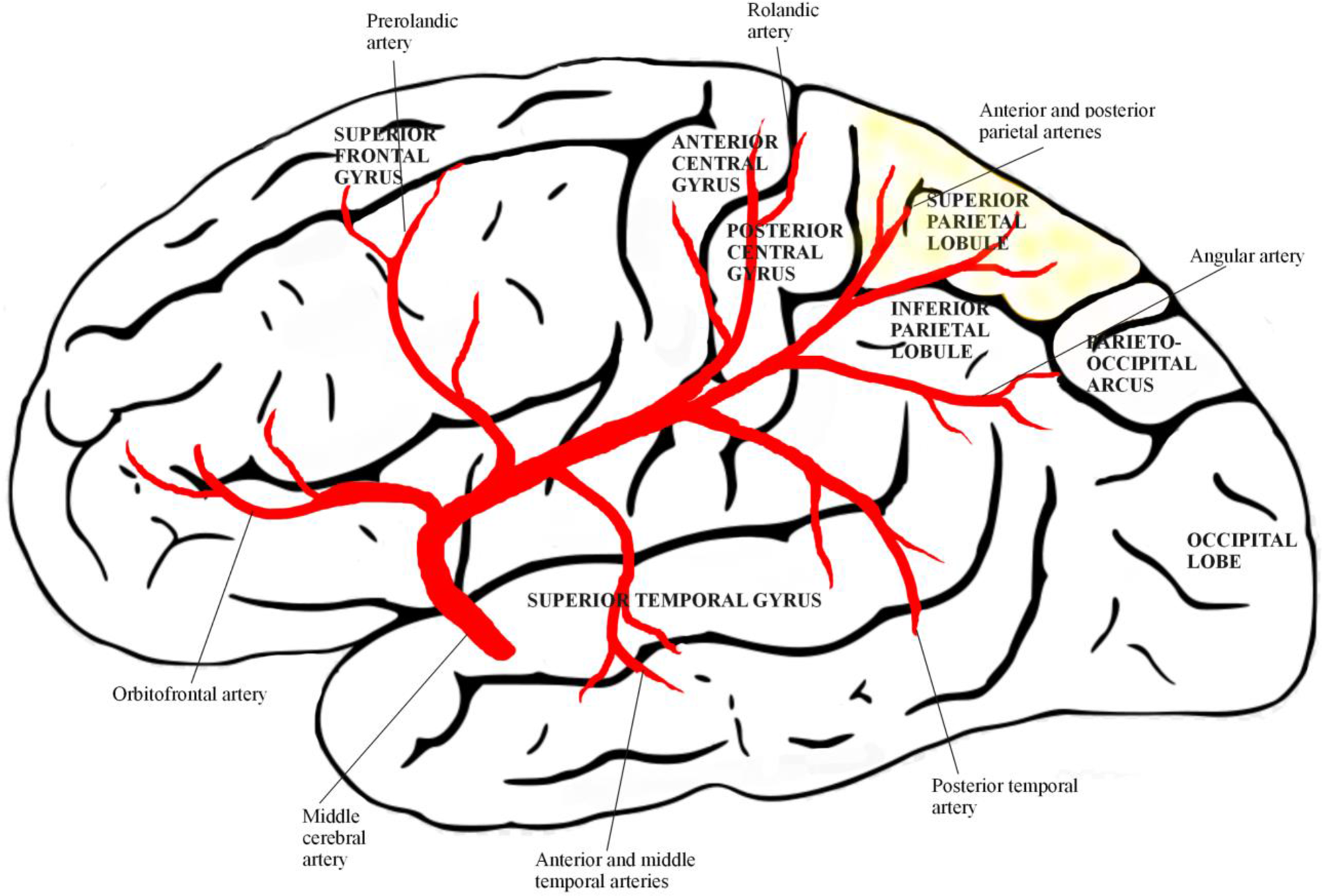
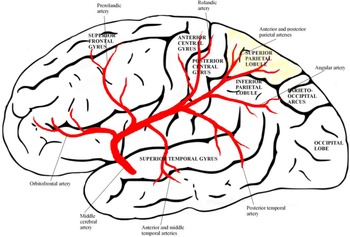
Figure 1: The superior parietal lobe is supplied by the posterior parietal branches of the medial cerebral artery.
Table 2: Demographic and clinical findings of patients with superior parietal lobule infarct
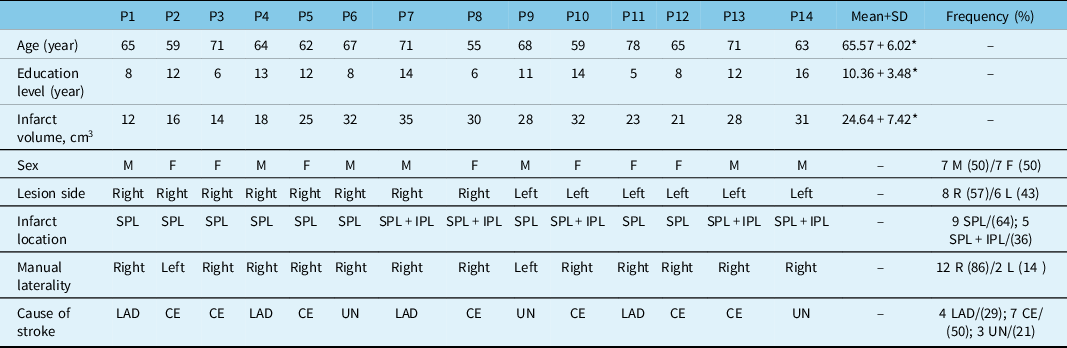
AD: antero-dorsal precuneus; CE: cardioembolism; LAD: large-artery disease (extracranial or intracranial atherosclerosis causing ≥ 50% luminal stenosis); UN: unknown; SPL: superior parietal lobule; IPL: inferior parietal lobule; M: male; F: female; R: right; L: left.
Values in parentheses are percentage of raws.
At stroke onset, there were miscellaneous neurological and behavioral complaints after lesions of the left or right hemisphere such as tactile size (57%), roughness (50%), shape (57%), texture (50%) discrimination disorders, astereognosia (36%) tactile memory of objects in space (14%), tactile feeling of strangeness (43%), tactile agnosia (7%), finger agnosia (7%), topographical disorientation (14%), left/right indistinction (21%), acalculia (21%), alexia (14%), and agraphia (7%) (Figure 2) (Tables 3 and 4). Among six patients with mild hemiparesis, 4 had right hemisphere stroke and 2 of them had anosognosia for hemiparesis. Motor deficits were improved in 2 weeks.
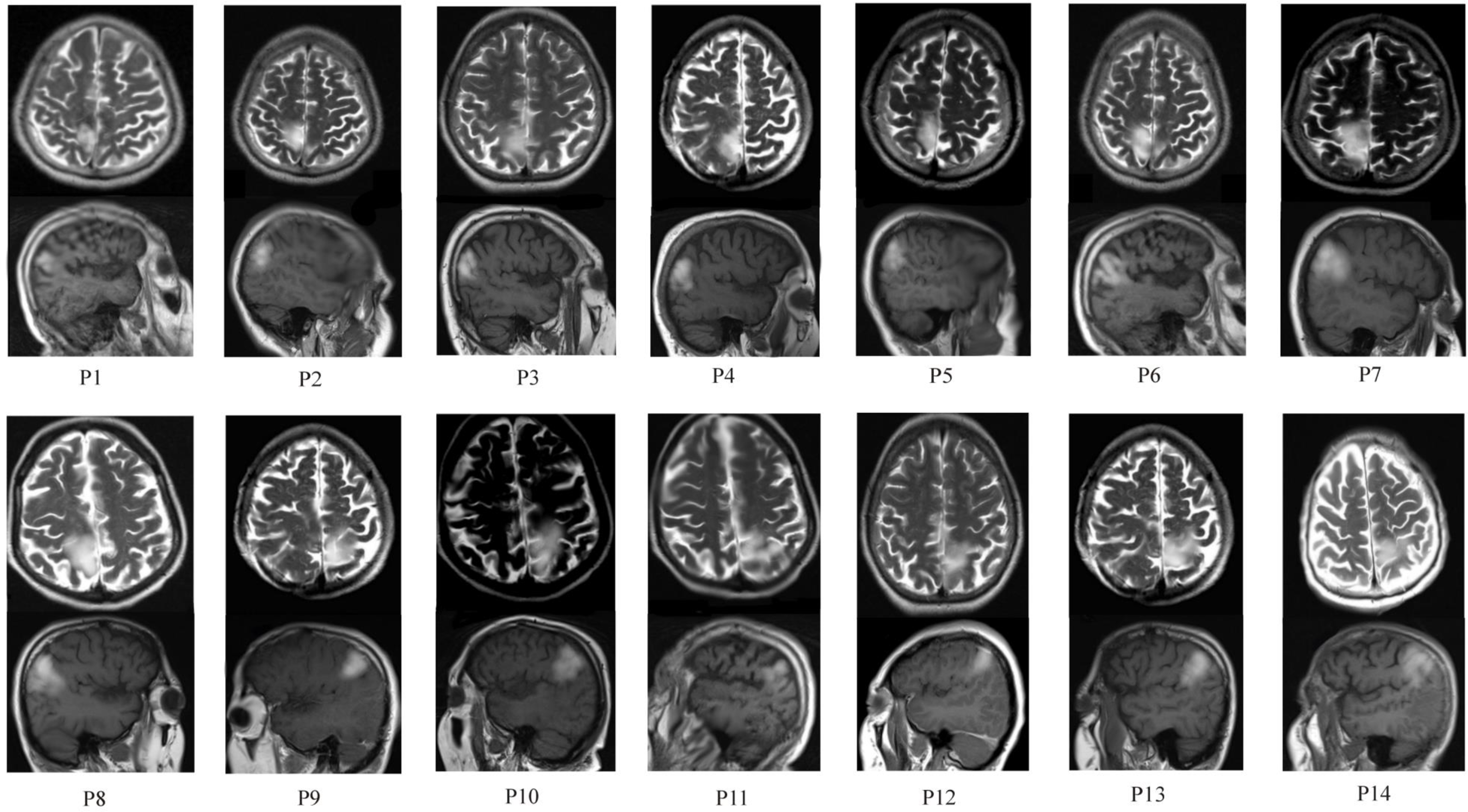
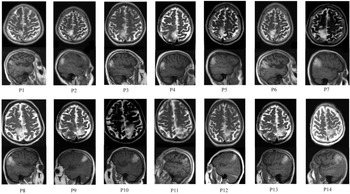
Figure 2: Axial and sagittal MRI images of the brain, showing high intensity in the anterior and posterior parts of the superior parietal lobule (SPL). Inferior parietal lobe (IPL) was involved partially in 5 patients (P7,P8,P10,P13,P14).
Memory Disorders (MDs)
In the acute phase of stroke, topographical memory was impaired in 2 patients (P7, P8) with right-sided lesions, characterized by loss of recognition of 10 important non-color photographs of outdoor topographical scenes. Episodic memory which carries information about personal events and episodes which are time and space-specific was impaired in 4 patients (P6, P8,P13, and P14). Four patients (P6, P7, P8, and P12) had semantic memory deficits characterized by disturbances of information and knowledge of the world and objects.
Visuospatial Disorders (VSDs)
Spatial disorientation, characterized by deficits to match two angled lines, was present in 2 patients (P7, P8). Visuospatial neglect demonstrated by line bisection test was found in 4 patients (P1, P4, P7, and P8). Visual extinction (29%) and motor neglect (14%) with underutilization of the upper extremity for tasks were found in patients with right hemisphere lesion. Optic ataxia was observed in one patient (P14) with superior and partial IPL stroke.
BSIDs
On the first day of stroke, ten patients had at least one BSDs (ranging from one to six). Allochiria (tactile) (7%), alien hand syndrome (7%), autotopagnosia for body parts (36%), autotopagnosia for sensory sensations (36%), fading limb (21%), supernumerary limb (7%), microsomatognosia (7%), macrosomatognosia (7%), hemiconcern syndrome (7%), tactile extinction (21%), anosodiaphoria (14%), xenomelia (7%), and invisible doppelgänger (7%). Patients with BSDs are presented in Table 4. Fading limb is related to the whole hemi-body for patients P3 and P7, the arm and hand for P8. Two patients (P6, P7) had anosodiaphoria, characterized by awareness of deficiency, but failed to fully comprehend this or its significance for functioning. Three patients with fading limb (P3, P7, and P8) had disturbance of “transparency” feeling of their limbs and had difficulty locating the contralesional part of the limbs in the space when they had the eyes closed. Patient 2 had autotopagnosia for sensory sensations, macrosomatognosia, perceiving the left parts of her body as disproportionately large and xenomelia characterized by a desire for the amputation of healthy left leg. Patient 3 had simultaneously fading limb and the feeling of the presence of his invisible Doppelgänger (Figure 3).
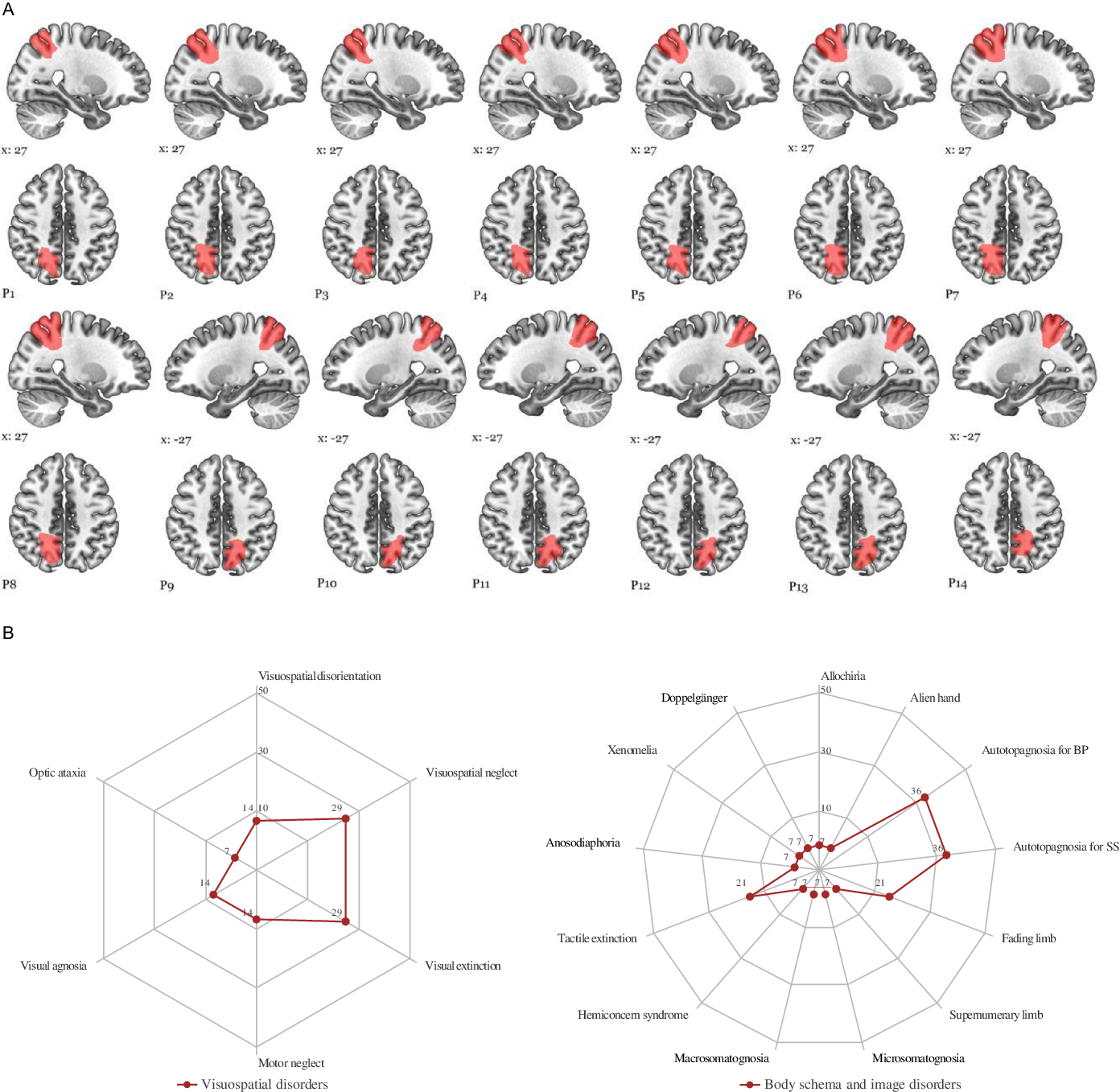
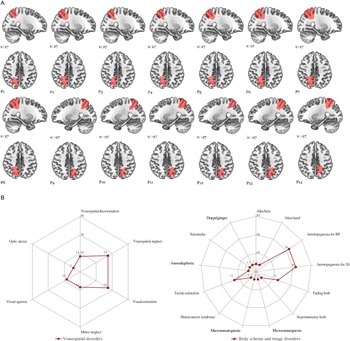
Figure 3: Schematic drawings of ischemic lesions. (A) Ischemic damaged sectors of the precuneus are plotted individually onto the standard MNI152 template using MRIcroGL. (https://www.mccauslandcenter.sc.edu/mricrogl/home). (B) Radar plot of visuospatial and body schema and image disorders observed in the first week after stroke. Values shown as percentage of cases. BP: autotopagnosia for body parts; SS: autotopagnosia for body sensations (see also Tables 2 and 3).
Table 3: Clinical findings of patients with superior parietal lobule infarct and frequency of findings in first week after stroke
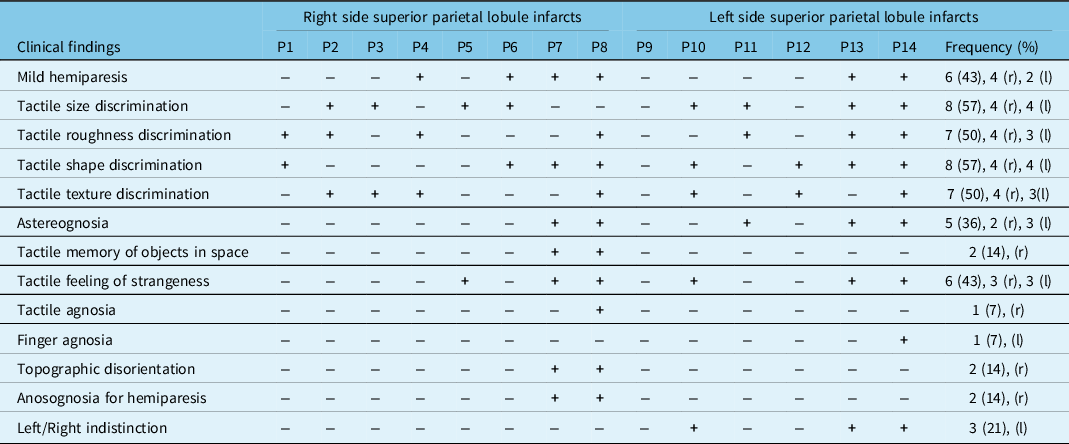
P4, P6, P7, P8, P13, P14 are patients with hemiparesis at the onset of stroke. r, right side; l: left side.
Table 4: Cognitive disorders of patients with superior parietal lobule infarct in the first week after stroke
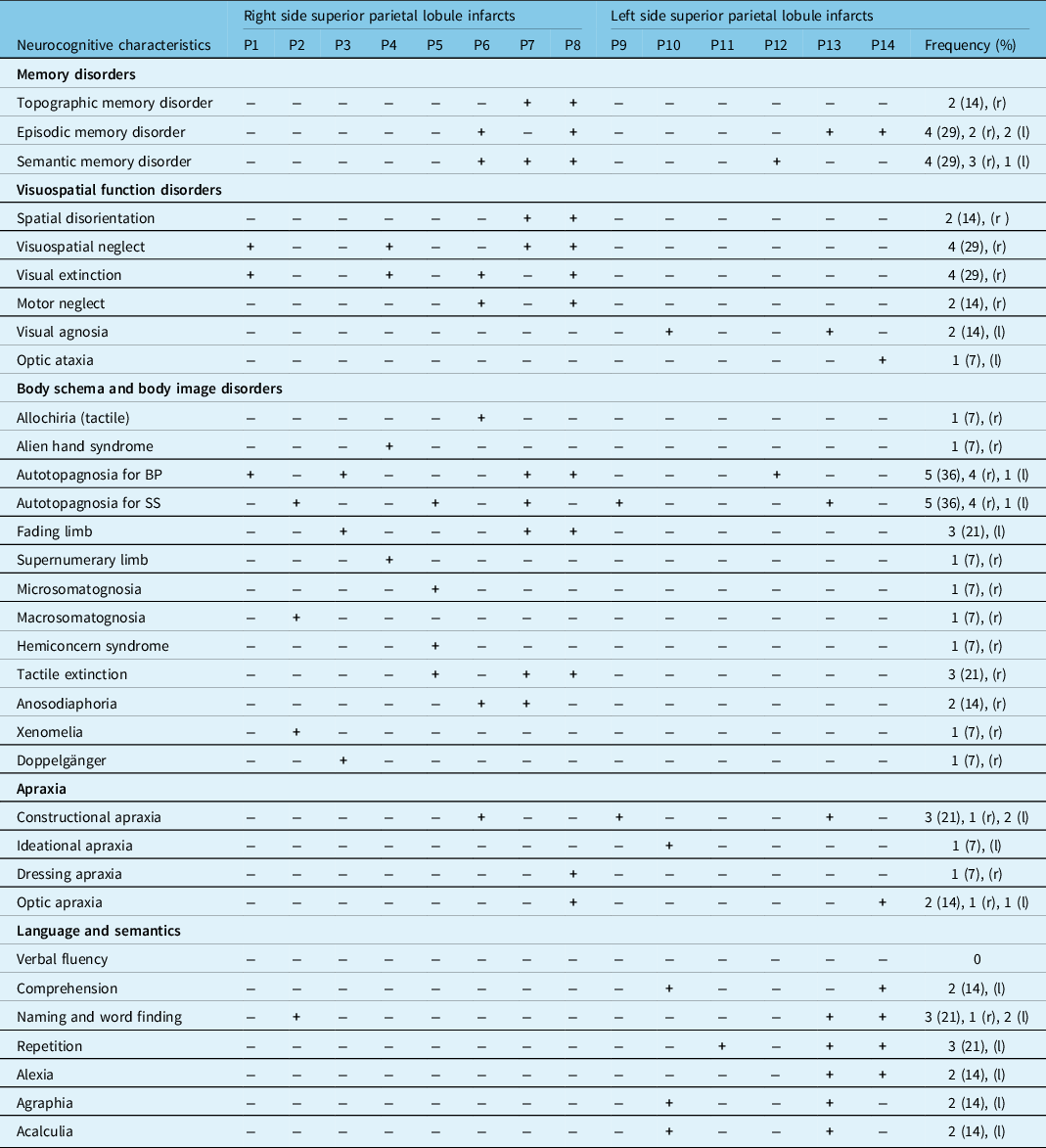
+: deficit (i.e. Z < 2 or score < cutoff); −: no deficit; BP: body parts; SS: sensory sensations; SD: first week after stroke; 3 M = three months after stroke. TMT: Trail Making Test; r: right side; l: left side. Spatial neglect was present in 7 patients, and one of them, P4, had left side lesion. Note that, for P1 and P2, the fading limb concerned the entire hemi-body; for P6, it concerned the leg while, and for P11, it concerned the hand and the leg. For P9, it concerned the entire hemi-body. For P7, the macrosomatognosia concerned the arm, and for P3, microsomatognosia involved the leg and the foot. For P1, 3, and 5, the autotopagnosia for BP involved the entire body part. Semantic matching and fluency deficit were present in the half of patients.
Verbal Disorders (VDs)
Verbal comprehension (14%), naming and word finding (21%), repetition (21%), reading (14%), writing (14%), calculation (14%) were impaired at stroke onset. Patient 13 had alexia with agraphia syndrome.
Other Neuropsychological Disorders
Constructional apraxia (21%), ideational apraxia (7%), and dressing apraxia (7%), especially in the post-stroke period, were also presented. Patient 14 had optic ataxia and apraxia with absence of voluntary, purposeful eye movement, and deficit in reaching visual goals. Fisher’s exact test showed that patients with memory disorders (P < 0.05) and VSDs (P < 0.05) had larger stroke volume (>25cm3) than those without these disorders. Although body schema disorders are seen in all right-sided lesions, verbal disorders were observed in 67% of left-sided lesions.
Discussion
This study showed the role of SPL in various types of cognitive and behavioral functions such as memory, body schema, and body image, visuospatial and linguistic functions. The current study identified that involvement of different areas of the SPL following ischemic stroke, typically the anterior or the posterior parts, and sometimes the whole SPL were likely to build the neural basis of these disorders.
Though precentral gyrus is normally associated with movement and postcentral gyrus with sensory function, in the meantime SPL is activated for integration of sensory stimuli. Transient paralysis of the limbs in six patients could be explained by the edematous effect of the infarct site or by transient hypoperfusion of adjacent areas. The SPL is responsible for the integration and differentiation of tactile stimuli from the postcentral gyrus, and its damage has been shown to impair these processes. No distinction was observed between the right and left hemispheres in tactile discrimination dysfunctions. However, our topographical analysis indicated that the SPL was likely to comprise the neural correlate of the VSDs, including spatial disorientation, spatial neglect, visual extinction, and motor neglect. Indeed, previous studies indicated that SPL plays a crucial role in regulating visuospatial attention and visuospatial functions resulting from unbalanced interactions between the bilateral frontoparietal networks and the interhemispheric parietal network. Reference Wu, Wang and Zhang22-Reference Leech, Kamourieh, Beckmann and Sharp24 It has been unraveled that the right SPL predominantly mediated visuospatial attention compared to left SPL. It is also worth noting that some patients had lesions extending toward the IPL, a border region between anterior and middle cerebral artery. Considering the known functions of these brain regions in spatially guided behavior and cross-modal integration, respectively, Reference Leech, Kamourieh, Beckmann and Sharp24,Reference Selemon and Goldman-Rakic25 damage to these regions, in principle, can lead to VSDs.
Quite recently, new studies demonstrated the selective role of the right parietal cortex in topographical memory. Reference Maguire and Cipolotti26,Reference della Rocchetta, Cipolotti and Warrington27 Their recognition memory performance for topographical scenes, unknown buildings, and landscapes was remarkably impaired. However, their spatial learning skill performance was preserved. The topographical memory impairment presented in this study was probably related to the dysfunction of the network between the SPL, IPL, extrastriate regions, bilateral occipitotemporal areas, and bilateral parahippocampal gyrus. Moreover, it is worth noticing that some patients experienced episodic and semantic memory disorders which are functionally distinct, though overlapping memory systems might be disturbed by damage of SPL whose interlinked selectively with cingulate and prefrontal cortices. Reference LaBar, Gitelman, Parrish and Mesulam28
It should be noted that various BSIDs related to SPL strokes were found in this study. It is possible that different aspects of BSIDs might be developed either by suppression of some tactile information as observed in alien hand and fading limb, and autotopagnosia for body parts, or by over or underestimation of the size of specific body parts as seen in micro- or macrosomatognosia and supernumerary limb. This is in line with the fact that BSIDs are linked to impaired sensorimotor integration. Reference Koenigs, Barbey, Bradley and Grafman29-Reference Weijers, Rietveld, Meijer and De Leeuw31 Selective disruption of these processes may lead to a fading limb, anosodiaphoria, doppelgänger as we presented here, three of them (P3, P7, and P8) had a large SPL infarct, extending toward IPL, experienced a sense of loss of contralesional limb when not under visual control. Indeed, the integration of both tactile and proprioceptive modalities from the contralesional body, or maintaining a representation of the current limb position, is critical for determining the location of a body member in space. Reference Vallar and Ronchi32-Reference Graziano34
It is also noted a patient with xenomelia who had desire for the amputation of upper left limb arose from right SPL stroke. This body integrity identity disorder probably resulted by inadequate activation of the right SPL leading to the unnatural situation in which the sufferer can feel the limb in question being touched without it actually incorporating into their body image, with a resulting desire for amputation. Reference McGeoch, Brang, Song, Lee and Huang35 On the contrary, hemiconcern syndrome which is characterized by concentration on the left side of the body and manipulation parts of the left arm, and leg with her right hand or foot, was seen in one patient.
Impaired language skills such as difficulties in understanding, word finding, naming, and repetition were found in patients with lesions extending to the IPL., suggesting that the left SPL and IPL in man may be crucial for the sensorimotor linguistic integration. This linguistic integration is running principally between the SPL and superior temporal gyrus which are connected by the middle longitudinal fascicle. Pure agraphia and agraphia plus alexia have also been noted in patients with lesions extending posteriorly. In fact, some authors Reference Auerbach and Alexander36,Reference Paolino, De Bastiani, Monetti, Boidrini and Rosati37 have described cases of pure agraphia clinically indistinguishable from ours, in which the left SPL is involved. The present cases would seem to confirm that SPL plays a role in writing and reading but, in our view, additional information is required before drawing conclusions on the nature of these findings.
This study had some limitations. Sample size is small, but it is uncommon for a stroke injury in the isolated parietal lobule without extension to other parts of the brain. The modern imaging technique, MRI, has made a significant contribution to showing the isolated lesion borders from the axial and coronal aspects. It should be noted that this study did not undertake specific experimental tasks and functional imaging studies. However, this study showed that ischemic lesions of the SPL can cause a variety of cognitive and behavioral disorders related to disruption of a broad network in a wider range of associative subcortical and cortical structures. Furthermore, we cannot completely exclude the possibility that ischemic adjacent cortices may also play a role in the development of cognitive deficits. For example, some patients (P7, P8, P10, P13, P14) had an infarction partially involving IPL. Given the established role of these brain areas in visuospatial functions, somatosensory processing, and language skills, ischemic damage to these areas can ultimately lead to a wide and diverse spectrum of cognitive impairment. It is worth noting that in some cases, the posterior SPL was also affected, which has reached connections with visuomotor system. Patients with optic ataxia and apraxia had ischemic lesions extending posteriorly, including the intraparietal sulcus and superior part of the IPL, or more often various parts of the SPL. The weak co-occurrence of optic ataxia, apraxia, and hemispatial neglect, and their different lesion sites, indicate a double dissociation between these symptoms. Reference Battaglia-Mayer and Caminiti14,Reference Karnath and Perenin38 Eventually, it seems that a lesion involving these structures may cause distinct but related visuomotor control disorders. Another limitation of our study is that MR images were taken after stroke, and control examinations were not available. Also, it should be noted that stroke volume was associated with some cognitive functions. However, it is difficult to establish an association between body schema disorders, verbal functions, and volume size due to the low number of cases and the fact that the disorders are part of a wide functional network.
The main cause of SPL ischemic lesions was embolism, which originated from atrial cardiopathy and carotid atherosclerotic plaques. Isolated lesions in the SPL could most likely be due to the fact that these small embolic particles damaged certain small areas.
To summarize, our findings after stroke suggest that the SPL plays a pivotal role in the regulation of memory, visuospatial abilities, body schema and body image processing, and language skills by interactions between the bilateral frontoparietal networks and the interhemispheric parietal network.
Data Sharing Statement
-
Individual deidentified participant data are available, including data dictionaries will be shared on request
-
The data were entered into the registry between April 2013 and December 2019.
-
Related documents such as study protocol and statistical analysis plan will be shared on request.
-
The data will be made available; analyses and mechanisms were performed by Hüseyin Nezih Özdemir M.D., explained in Methodology section.
Acknowledgements
We are grateful to all the patients who took part in the study. The authors would especially like to thank the clinical staff at the Department of Neurology, Ege University, School of Medicine, İzmir Türkiye. We would like to acknowledge the contribution of Mr Hasan Arslan and Ms Günay Çoşkun toward the analysis of the neurocognitive tests.
Conflict of Interest
The authors have no conflict of interest to disclose.
Statement of Authorship
Principal author: Emre Kumral M.D.
Study concept or design: Emre Kumral M.D., Fatma Ece Bayam M.D.
Acquisition of data: Hüseyin Nezih Özdemir M.D.
Analysis or interpretation of data: Hüseyin Nezih Özdemir M.D.
Statistical analysis: Emre Kumral M.D.
Study supervision or coordination: Emre Kumral M.D., Fatma Ece Bayam M.D.
Ethical Committee Approval
Ege University Medical Ethical Committee was approved this study following the principles outlined in the Helsinki Declaration before starting the study (2010/101).