Myasthenia gravis (MG) is an autoimmune disorder of the neuromuscular junction, which results in weakness that can range from mild to life threatening. Confirmation of a clinical diagnosis of MG depends on electrophysiological studies and serological testing for autoantibodies directed against postsynaptic components of the neuromuscular junction.
The two autoantibodies most commonly used to aid in the diagnosis of MG are anti-acetylcholine receptor (AChR) antibodies and anti-muscle-specific tyrosine kinase (MuSK) antibodies. AChR antibodies are positive in approximately 85% of generalized MG casesReference Meriggioli and Sanders 1 ; however, fewer patients with ocular MG are positive for these autoantibodies than generalized MG patients. MuSK antibodies are positive in approximately 40% of patients with MG who are AChR–, although there appears to be geographical variation, with lower prevalence in populations a greater distance from the equator.Reference Deymeer, Gungor-Tuncer and Yilmaz 2 - Reference Vincent, Leite and Farrugia 4 In approximately 9% of cases of MG, both AChR and MuSK antibodies may be negative at presentation, and such patients are referred to as double-seronegative MG (dSNMG).Reference Zhang, Tzartos and Belimezi 5 One study has reported that with a follow-up period of 12 months, rates of dSNMG may decrease to 5%.Reference Chan, Lachance, Harper and Lennon 6 The dSNMG patients are generally assumed to harbor autoantibodies to other components of the postsynaptic apparatus. In addition to being diagnostically useful, the identification of other autoantibodies may provide prognostic information and help guide treatment.
Low-density lipoprotein receptor-related protein 4 (LRP4) is a membrane protein that serves as a receptor for agrin in the neuromuscular junction. The binding of agrin to LRP4 enables the activation of MuSK, which results in the phosphorylation of cortactin, which in turn mediates the clustering of AChR.Reference Madhavan, Gong, Ma, Chan and Peng 7 AChR clusters at sites on the muscle membrane where synapses from motor neurons occur (as opposed to parts of the muscle membrane at which no synapses occur). This AChR clustering results in high receptor availability to synaptic acetylcholine and thereby facilitates muscle excitability.Reference Sanes and Lichtman 8
Because of its role in the neuromuscular junction, autoantibodies to LRP4 have been investigated in MG. Animal studies have previously indicated that anti-LRP4 antibodies may induce a myasthenic state.Reference Shen, Lu and Zhang 9 Anti-LRP4 antibodies may be detected through different techniques, including luciferase immunoprecipitation, enzyme-linked immunosorbent assay (ELISA), and newer cell-based assays (CBAs). Estimates of the prevalence of anti-LRP4 antibodies in dSNMG have varied widely (see the following section); some of this variation may be explained by the methods of detection used.
The aim of this paper is to identify studies in which the frequency of positivity for anti-LRP4 antibodies has been assessed in patients with dSNMG, or in which the clinical characteristics (epidemiology, clinical features, management) of LRP4+ dSNMG patients have been reported.
Methods
A search of the databases PubMed, EMBASE, Medline, and Scopus was conducted on January 14, 2017, and included results published since the respective commencements of the databases. The searches used the Medical Subject Headings “myasthenia gravis” and “low-density lipoprotein receptor-related protein 4” or “LRP4.” Results were then limited to those published in English.
Following the application of this language restriction, the titles and abstracts of the remaining publications were viewed to determine if they met the inclusion criteria. The following inclusion criteria were used: (1) involved human subjects, including individual case reports (excluding reviews); (2) involved patients with MG; (3) assessed the presence of autoantibodies in these MG patients; (4) the antibodies assessed included LRP4; (5A) presented information on the percentage of individuals negative for AChR and MuSK who were positive for LRP4 or (5B) presented information on the characteristics (epidemiology, clinical features, electromyographic findings or management) of dSNMG patients (negative for AChR and MuSK) who were LRP4+; and (6) were available in full-text.
Accordingly, studies presenting information on seropositive MG patients, or patients with other neurological diseases positive for anti-LRP4 antibodies were excluded.
Articles that appeared likely to fit inclusion criteria based on title or abstract were then reviewed in full-text. If it could not be determined whether an article met inclusion criteria based on the title or abstract, it was also retrieved and viewed in full text before inclusion or exclusion. Reference lists of included articles were searched for other potentially eligible studies. Eligibility determination and data extraction were performed in duplicate by SB and PK using a standardized form. Inconsistencies were resolved with discussion until consensus was reached.
Results
The initial search produced 367 articles, which was reduced to 313 when an English language filter was applied. The titles and abstracts of these articles were reviewed, yielding 41 articles to be viewed in full-text. 14 papers met the inclusion criteria (Figure 1).
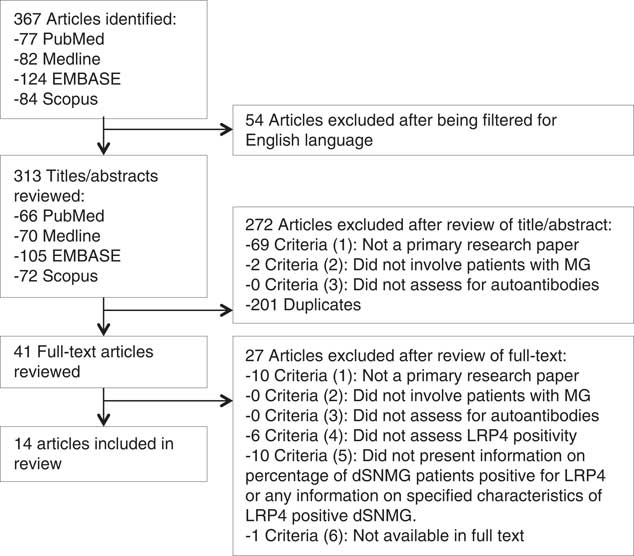
Figure 1 Flowchart detailing results from the search strategy and application of inclusion and exclusion criteria for a review of articles assessing the frequency and clinical implications of anti-LRP4 positivity in dSNMG.
Of the 14 included articles, there were ten cross-sectional studies,Reference Zhang, Tzartos and Belimezi 5 , Reference Cossins, Belaya and Zoltowska 10 - Reference Zisimopoulou, Evangelakou and Tzartos 18 three case series, 19 - Reference Zouvelou, Zisimopoulou and Rentzos 21 and one case report.Reference Beck, Yabumoto and Baba 22 The majority (11) of these papers used CBA to detect LRP4 antibodies.Reference Cossins, Belaya and Zoltowska 10 - Reference Gallardo, Martinez-Hernandez and Titulaer 12 , Reference Marino, Scuderi and Samengo 14 - Reference Zouvelou, Zisimopoulou and Rentzos 21 Other methods used were luciferase immunoprecipitationReference Higuchi, Hamuro, Motomura and Yamanashi 13 , Reference Beck, Yabumoto and Baba 22 and ELISA.Reference Zhang, Tzartos and Belimezi 5
The primary limitation for the majority of the studies was the small numbers of LRP4+ dSNMG patients (Table 1).Reference Cossins, Belaya and Zoltowska 10 , Reference Rodríguez Cruz, Al-Hajjar and Huda 11 , Reference Higuchi, Hamuro, Motomura and Yamanashi 13 - Reference Nikolic, Bojic, Rakocevic Stojanovic, Basta and Lavrnic 15 , 19 Sample size varied greatly among the studies, from 635 dSNMG (yielding 119 LRP4+ patients) to an individual case report.Reference Zisimopoulou, Evangelakou and Tzartos 18 , Reference Beck, Yabumoto and Baba 22 Six of the ten studies explicitly stated that the outcome assessor(s) were blinded.Reference Zhang, Tzartos and Belimezi 5 , Reference Gallardo, Martinez-Hernandez and Titulaer 12 , Reference Nikolic, Bojic, Rakocevic Stojanovic, Basta and Lavrnic 15 - Reference Zisimopoulou, Evangelakou and Tzartos 18 Only two of the ten studies stated the number of outcome assessors.Reference Gallardo, Martinez-Hernandez and Titulaer 12 , Reference Nikolic, Bojic, Rakocevic Stojanovic, Basta and Lavrnic 15 Five of the cross-sectional studies reported the methods by which a diagnosis of MG was established.Reference Zhang, Tzartos and Belimezi 5 , Reference Rodríguez Cruz, Al-Hajjar and Huda 11 , Reference Higuchi, Hamuro, Motomura and Yamanashi 13 , Reference Pevzner, Schoser and Peters 16 , Reference Zisimopoulou, Evangelakou and Tzartos 18
Table 1 Results from studies that have assessed the frequency of anti-LRP4 positivity in dSNMG patients
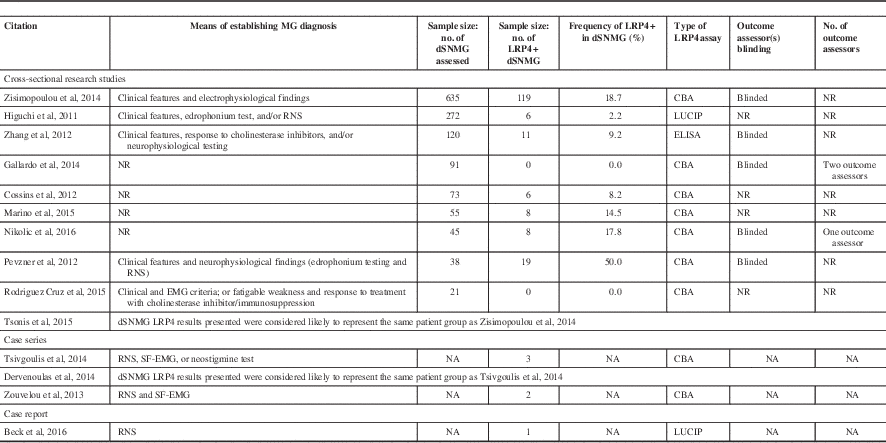
LUCIP=Luciferase-reporter immunoprecipitation; NA=not available; NR=not reported; RNS=repetitive nerve stimulation; SF-EMG=single-fiber EMG.
The data presented on the dSNMG LRP4+ group by Tsonis et alReference Tsonis, Zisimopoulou and Lazaridis 17 were considered likely to be the same patient population as that presented in Zisimopoulou et alReference Zisimopoulou, Evangelakou and Tzartos 18 because of the similarities between clinical features and thymic pathology and that the same research group performed the two studies. The results presented in the abstract by Dervenoulas et al 19 were considered likely to represent the same patients as those reported in Tsivgoulis et al.Reference Tsivgoulis, Dervenoulas and Kokotis 20 Accordingly the results for the LRP4+ dSNMG groups from Tsonis et al and Dervenoulas et al are not presented independently in the following section.
Frequency of Anti-LRP4 Antibody Positivity in dSNMG
There was a wide range of results regarding the frequency of LRP4 antibodies in dSNMG patients (Table 1), from 50% in Pevzner et al (n=38 dSNMG) to 2% in Higuchi et al (n=272 dSNMG).Reference Higuchi, Hamuro, Motomura and Yamanashi 13 , Reference Pevzner, Schoser and Peters 16 There were also two studies that failed to identify any LRP4+ dSNMG patients: Gallardo et alReference Rodríguez Cruz, Al-Hajjar and Huda 11 (n=91 dSNMG) and Cruz et alReference Gallardo, Martinez-Hernandez and Titulaer 12 (n=21 dSNMG).
The largest study to date that has assessed patients from multiple countries did not identify an obvious pattern in the geographical variation.Reference Zisimopoulou, Evangelakou and Tzartos 18 For example, this study identified a prevalence of 32.8% in Poland (19/58) and 7% in Norway (3/43). This is in contrast to MuSK antibodies, which appear to have increasing prevalence closer to the equator.4
Differences in the LRP4 antibody assays may also account for the varying prevalence. CBA appear, in general, to have a higher sensitivity for detecting anti-LRP4 antibodies. CBA studies have reported frequencies of 50%, 18.7%, 17.8%, 14.5%, and 8.2%.Reference Cossins, Belaya and Zoltowska 10 , Reference Marino, Scuderi and Samengo 14 - Reference Pevzner, Schoser and Peters 16 , Reference Zisimopoulou, Evangelakou and Tzartos 18 However, the two studies that failed to identify any LRP4+ dSNMG also both used CBA,Reference Rodríguez Cruz, Al-Hajjar and Huda 11 , Reference Gallardo, Martinez-Hernandez and Titulaer 12 although one of these studies had a sample size of only 21.Reference Rodríguez Cruz, Al-Hajjar and Huda 11 Zhang et al used ELISA and found a prevalence of 9.2%.Reference Zhang, Tzartos and Belimezi 5 Higuchi et al used luciferase immunoprecipitation and found a prevalence of 2.2%.Reference Higuchi, Hamuro, Motomura and Yamanashi 13
Epidemiology of LRP4+ dSNMG
Three cross-sectional studies indicated that patients who have LRP4+ dSNMG seem to be more likely to have a younger age of onset of the disease and are more likely to be female than LRP4- dSNMG.Reference Marino, Scuderi and Samengo 14 , Reference Pevzner, Schoser and Peters 16 , Reference Zisimopoulou, Evangelakou and Tzartos 18
The study with the largest sample of LRP4+ dSNMG patients (119 patients) identified an average age of onset of 34.9 years. This study also reported that 84% of these patients had an onset of the disease earlier than the age 50.Reference Zisimopoulou, Evangelakou and Tzartos 18 Other studies with smaller sample sizes have supported this result. For example, Marino et alReference Marino, Scuderi and Samengo 14 found that 87.5% of LRP4+ dSNMG patients had the onset of their disease before age 50, compared with 74.5% of LRP4– patients (in a sample including 8 LRP4+ and 55 LRP4– dSNMG patients). Similarly, in the initial sample in Pevzner et al, all seven of the LRP4+ dSNMG patients experienced disease onset before 50 years of age Reference Pevzner, Schoser and Peters 16 ; however, new-onset cases of LRP4+ dSNMG have been reported in individuals from ages 26 to 83.Reference Higuchi, Hamuro, Motomura and Yamanashi 13 , Reference Tsivgoulis, Dervenoulas and Kokotis 20
Zisimopolou et al found LRP4+ dSNMG to be more common in women (female:male [F:M] ratio of 2.5:1) compared with LRP4– dSNMG.Reference Zisimopoulou, Evangelakou and Tzartos 18 This was supported by Marino et al, in which the tendency for LRP4+ to occur in women was stronger (LRP4+dSNMG F:M 7:1 vs LRP4- dSNMG F:M 2.1:1), although with a much smaller sample size (n=8 LRP4+ dSNMG).Reference Marino, Scuderi and Samengo 14 Nikolic et al identified a higher proportion of male LRP4+ dSNMG patients than other studies (F:M 1.7:1), but had no LRP4– dSNMG comparator group.Reference Nikolic, Bojic, Rakocevic Stojanovic, Basta and Lavrnic 15 Pevzner et alReference Pevzner, Schoser and Peters 16 2012 found the opposite trend, with LRP4– dSNMG being more common in females; however, the sample size was small (n=7 LRP4+dSNMG and n=6 LRP4– dSNMG).
There has been significant variation in the prevalence of LRP4+ in dSNMG by the country of residence of the patient (see previous section).
Clinical Features of LRP4+ dSNMG
Four studies found that LRP4+ dSNMG often presents with isolated ocular weakness and is most commonly mild in severity (Myasthenia Gravis Foundation of America [MGFA] I-II).Reference Marino, Scuderi and Samengo 14 , Reference Nikolic, Bojic, Rakocevic Stojanovic, Basta and Lavrnic 15 , Reference Zisimopoulou, Evangelakou and Tzartos 18 , Reference Tsivgoulis, Dervenoulas and Kokotis 20 One case series described patients with LRP4+ dSNMG presenting with isolated ptosis and diplopia, with the duration of symptoms ranging from 12 to 36 months.Reference Tsivgoulis, Dervenoulas and Kokotis 20 Nikolic et al and Zisimopoulou et al identified a significant proportion of LRP4+ dSNMG patients presenting with isolated ocular symptoms (MGFA I 50% and 29.9%, respectively).Reference Nikolic, Bojic, Rakocevic Stojanovic, Basta and Lavrnic 15 , Reference Zisimopoulou, Evangelakou and Tzartos 18 In some case reports, LRP4+ dSNMG initially presented with primarily neck extensor weakness.Reference Zouvelou, Zisimopoulou and Rentzos 21 , Reference Beck, Yabumoto and Baba 22
Moderate to severe disease (MGFA III-IV) appears to be less common than mild disease in LRP4+ dSNMG. The largest study (Zisimopoulou et al) had clinical data on 67 patients with LRP4+, of which 57 (85.1%) had MGFA I-II disease and ten (14.9%) had MGFA III-IV disease.Reference Zisimopoulou, Evangelakou and Tzartos 18 Two smaller studies found rates of MGFA I-II disease of 75% and 62.5% (vs MGFA III-IV 25% and 25%, respectively).Reference Marino, Scuderi and Samengo 14 , Reference Nikolic, Bojic, Rakocevic Stojanovic, Basta and Lavrnic 15 Higuchi et al and Pevzner et al reported a different pattern to that described previously, with generalized disease and moderate severity being a more common presentation than isolated ocular weakness and mild disease; however, these studies are small (n=6 and 7 LRP4+ dSNMG cases, respectively).Reference Sanes and Lichtman 8 , Reference Higuchi, Hamuro, Motomura and Yamanashi 13
Although uncommon, cases have been documented of LRP4+ dSNMG requiring intubation (MGFA V).Reference Higuchi, Hamuro, Motomura and Yamanashi 13 , Reference Nikolic, Bojic, Rakocevic Stojanovic, Basta and Lavrnic 15 , Reference Beck, Yabumoto and Baba 22
EMG in LRP4+ dSNMG
Only one paper specifically studied electromyography (EMG) findings in LRP4+ dSNMG patients. It was found that repetitive nerve stimulation was infrequently abnormal (abnormal in at least one nerve-muscle pair in only 12.5%) and that jitter values were lower than in the AChR+ and MuSK+ MG patients.Reference Marino, Scuderi and Samengo 14
Thymic Pathology in LRP4+ dSNMG
Zisimopolou et al presented the most comprehensive assessment of thymic changes, with hyperplasia in 13/42 (31%), an involuted thymus in 12/42 (28.6%), and thymus atrophy in 3/42 (7.1%), with all of the remaining being normal. Note that these 42 individuals for whom thymus pathology was available form a small subset of the patients involved in this study, and it is unclear whether they differed from the other patients in terms of age, gender, or presentation. However, it was reported that thymic abnormalities appear to be less frequent in the LRP4+ dSNMG patients than in AChR antibody positive MG patients (p < 0.05).Reference Zisimopoulou, Evangelakou and Tzartos 18
There was only one reported instance in the identified publications of an LRP4+ dSNMG patient having a thymoma.Reference Marino, Scuderi and Samengo 14 Higuchi et al reported that there was no evidence of thymoma in the six dSNMG LRP4+ patients that they identified.Reference Higuchi, Hamuro, Motomura and Yamanashi 13 Tsivgoulis et al reported no thymoma or thymic hyperplasia in any of the three patients in their case series.Reference Tsivgoulis, Dervenoulas and Kokotis 20 The patient reported on in Beck et al was found to have no evidence of thymoma on chest computed tomography.Reference Beck, Yabumoto and Baba 22 Zouvelou et al observed residual thymic tissue, which revealed thymus hyperplasia on thymectomy.Reference Zouvelou, Zisimopoulou and Rentzos 21
Treatment of LRP4+ dSNMG
Several cross-sectional studies and case series reported that the majority of patients with LRP4+ dSNMG responded well to cholinesterase inhibitors (such as pyridostigmine) and that, when used, the response to prednisone was good.Reference Pevzner, Schoser and Peters 16 , Reference Zisimopoulou, Evangelakou and Tzartos 18 , Reference Tsivgoulis, Dervenoulas and Kokotis 20 , Reference Zouvelou, Zisimopoulou and Rentzos 21 Zisimopoulou et al reported that 40/52 (76.9%) LRP4+ dSNMG patients had a good response to pyridostigmine and 7/52 (13.5%) had a moderate response. Similarly, they found that with prednisone 28/39 (71.8%) had a good response and 7/39 (18%) a moderate response.Reference Zisimopoulou, Evangelakou and Tzartos 18 However, there are several reported instances of further treatments, such as azathioprine, intravenous immunoglobulin, plasma exchange, tacrolimus, and thymectomy being required to control symptoms.Reference Nikolic, Bojic, Rakocevic Stojanovic, Basta and Lavrnic 15 , Reference Zisimopoulou, Evangelakou and Tzartos 18 , Reference Beck, Yabumoto and Baba 22 The outcomes reported in the Zisimopoulou et al study suggest that many LRP4+ dSNMG patients have good outcomes, with complete or pharmacologic remission in 34.6% (19/55), minimal manifestations in 5.5% (3/55), improved symptoms in 38.2% (21/55), and unchanged or worse in 16.4% (9/55) and 5.5% (3/55), respectively.Reference Zisimopoulou, Evangelakou and Tzartos 18
Discussion
We have reviewed the literature reporting the prevalence of anti-LRP4 antibodies in patients with dSNMG and the implications of anti-LRP4 positivity for such patients. A wide range of anti-LRP4 antibody positivity in dSNMG has been reported, which may reflect geographical location, assay technique, or other factors. Patients with dSNMG who are LRP4+ appear to be more likely to have early disease onset and to be female than LRP4– dSNMG patients. LRP4+ dSNMG patients seem likely to have mild disease, particularly with isolated ocular weakness, although more severe manifestations, including respiratory failure, are reported. Electrophysiological abnormalities appear to be milder, and thymoma is uncommon in anti-LRP4+ dSNMG patients. The majority of LRP4+ dSNMG patients appear to respond well to pyridostigmine, prednisone, or both; however, some patients have required long-term immunosuppression. There is little evidence to guide the use of thymectomy in LRP4+ dSNMG patients.
We limited our review to anti-LRP4 positivity in dSNMG patients only, and thus cannot draw detailed conclusions about anti-LRP4 positivity in other groups, such as seropositive MG and other neurological diseases. Five of the studies that were identified in this review also assessed anti-LRP4 status in seropositive (for anti-AChR or anti-MuSK) MG patients. Reports on patients who are seropositive for anti-AChR or anti-MuSK in addition to anti-LRP4 (dSNMG) suggest that double seropositive patients may have more generalized and severe disease than patients who are seropositive for one autoantibody alone.Reference Zisimopoulou, Evangelakou and Tzartos 18 , Reference Tsivgoulis, Dervenoulas, Tzartos, Zompola, Papageorgiou and Voumvourakis 23 It has been found that, generally, there are low rates of anti-LRP4 positivity in patients positive for another MG autoantibody, ranging from 0% to 10% (seropositive MG patient sample sizes, 97-174).Reference Zhang, Tzartos and Belimezi 5 , Reference Higuchi, Hamuro, Motomura and Yamanashi 13 , Reference Zisimopoulou, Evangelakou and Tzartos 18 However, there is also variation in the frequency of LRP4+ in seropositive MG because two studies identified rates of LRP4+ in AChR/MuSK seropositive patients of up to 22% (although with comparatively smaller sample sizes of 41-46 seropositive MG patients).Reference Marino, Scuderi and Samengo 14 , Reference Nikolic, Bojic, Rakocevic Stojanovic, Basta and Lavrnic 15 As for seronegative MG, antibody assay sensitivity may contribute to variability in the results of different studies assessing the frequency of autoantibody positivity in seropositive MG. A study published after the systematic search for this review was conducted has reported that in previously seronegative patients, highly sensitive radioimmunoassays and CBA can identify autoantibodies in an additional 37% of patients (30/81).Reference Hong, Zisimopoulou and Trakas 24
There have also been documented cases of anti-LRP4 positivity in diseases other than MG. The diseases in which anti-LRP4 antibodies have been identified include polymyositis,Reference Marino, Scuderi and Samengo 14 neuromyelitis optica,Reference Zhang, Tzartos and Belimezi 5 multiple sclerosis,Reference Zisimopoulou, Evangelakou and Tzartos 18 and amyotrophic lateral sclerosis (ALS).Reference Takahashi, Noto and Makita 25 , Reference Tzartos, Zisimopoulou and Rentzos 26 In particular, it has been reported that anti-LRP4 may be positive in up to 23.4% of ALS patients.Reference Takahashi, Noto and Makita 25 , Reference Tzartos, Zisimopoulou and Rentzos 26 The study that produced this result used CBAs in a sample of 104 ALS patients.Reference Tzartos, Zisimopoulou and Rentzos 26 This finding has implications for the specificity of anti-LRP4 for the diagnosis of dSNMG, particularly given that ALS may be a part of the differential diagnosis for patients presenting with weakness.
There have been some investigations of anti-LRP4 antibody subtypes, although none that specifically focused on dSNMG patients. Rather, these studies either did not specify whether the subjects were dSNMG or seropositive, or included both categories of MG patients in the same analysis. The anti-LRP4 antibodies identified were primarily immunoglobulin (Ig) G Reference Higuchi, Hamuro, Motomura and Yamanashi 13 and IgG2.Reference Zisimopoulou, Evangelakou and Tzartos 18 This is similar to anti-AChR, which is primarily IgG1,Reference Lefvert, Cuénoud and Fulpius 27 and in contrast to anti-MuSK, which is most commonly IgG3 or IgG4.Reference McConville, Farrugia and Beeson 28 - Reference Niks, van Leeuwen and Leite 30 This finding implicates anti-LRP4 as possibly playing a role in activating the complement cascade and resulting in neuromuscular junction dysfunction via this pathway.
We acknowledge that the exclusion of non-English language publications is also a potential limitation of this review. The possibility of publication bias or selective outcome reporting may also influence our conclusions.
Future research in this area should clearly define the criteria by which participants are diagnosed with MG, endeavor to standardize laboratory procedures between different geographic locations and assess larger samples of dSNMG patients. This may help to clarify the true frequency of anti-LRP4 antibodies in dSNMG. Further research into the role of thymectomy for LRP4+ dSNMG patients is required.
Conclusion
Seronegative MG is an ongoing diagnostic challenge. Reported anti-LRP4 antibody prevalence varies widely between studies. Characteristics of reported LRP4+ dSNMG patients include younger age at onset, female predominance, mild disease severity (frequently isolated ocular weakness), and a good response to pyridostigmine, prednisone, or both.
Acknowledgments and Funding
None.
Disclosures
None.
Statement of Authorship
SB and PK were involved in the development of the concept for the project, eligibility determination, data extraction, and manuscript preparation. CC was involved in the development of the concept for the project and manuscript preparation.






