Essential tremor (ET) is a chronic neurologic disorder and is the most common cause of tremor in adults.Reference Louis and Ferreira 1 In the recent literature on ET, there has been an improved understanding of the motor features of the illness and an increasing appreciation of non-motor features.Reference Louis 2 The identification of non-motor features such as mild cognitive impairment, depression, anxiety, sleep disturbances, hearing impairment, and possible olfactory dysfunction suggests that ET is not a monosymptomatic disorder.Reference Jhunjhunwala and Pal 3 , Reference Chandran and Pal 4 In terms of motor features, specific features for tremor of the limbs, impairment in tandem gait, and cerebellar type oculomotor abnormalities have been described. The phenotypic expression of these features varies between patients. Pathologically, the majority of patients with ET have Purkinje cell loss and an increase in Purkinje cell torpedoes in the cerebellum as compared with matched controls.Reference Louis, Faust and Vonsattel 5 Less frequently, atypical brainstem Lewy bodies have been found.Reference Louis, Faust and Vonsattel 5 These findings have not been corroborated by other investigators.Reference Rajput, Robinson, Rajput and Rajput 6 , Reference Symanski, Shill and Dugger 7 The heterogeneity of clinical features and pathology raises the possibility that ET is not a single entity, but a family of diseases.Reference Louis 2
The characteristic clinical feature of ET is a bilateral postural with or without kinetic tremor of the hands.Reference Elble 8 Tremor may also involve other parts such as head, neck, jaw, voice, and tongue.Reference Elble 8 Epidemiological studies have shown that patients with head tremor (HT) are more likely to be older and female.Reference Louis 9 In addition, there is preliminary evidence from imaging studies to suggest that ET patients with and without HT (HT+ and HT−) differ.Reference Louis 2 We further explored the hypothesis that ET is a family of diseases by examining whether ET patients who were HT+ differed from HT− patients based on demographic and clinical variables.
Methods
This study was conducted at the Department of Neurology, National Institute of Mental Health and Neurosciences, Bangalore, India. Over a period of six years (2009-2014), patients with ET were recruited from the neurology outpatient and movement disorders clinic. Written informed consent was obtained from all participants. They were enrolled in several clinical studies, which were approved by the Institute Ethics Committee. All patients were evaluated by a single movement disorders specialist (PKP). The diagnosis of ET was established using the National Institutes of Health collaborative genetic criteria.Reference Carranza, Snyder, Elble, Boutzoukas and Zesiewicz 10 , Reference Chouinard, Louis and Fahn 11 Demographic parameters such as gender, age, age at onset (AAO), presence of family history, and chronology of body part involvement were documented. Collateral histories from accompanying relatives were also obtained. For analysis, decade-wise grouping of patients was done based on AAO. HT was assessed in patients while seated comfortably and it was documented as either present or absent. HT, which was irregular and jerky in nature, was diagnosed as dystonic head tremor and excluded. Upper limb tremor was assessed with the hands at rest on the lap, with upper limbs extended at the elbow and outstretched in front of the patient, with upper limbs flexed at the elbow and held in front of the chest with fingers facing each other but not touching, and while performing the finger nose test. Lower limb tremor was examined with the patient seated in a chair, while lying down in bed, with lower limbs lifted off the bed with flexion at the hip, and while performing the knee heel test. Voice tremor in the patients with ET was assessed by sustained phonation and by careful observation while conversing with the examiner. Trunk tremor was assessed when the patients were comfortably seated and later when standing. Any rhythmic sidewise tremulousness of the trunk was considered as trunk tremor, which was documented as either present or absent.Reference Rivest and Marsden 12 Subjects with dystonic posturing of the limbs were excluded. However, subjects with subtle abnormal posturing of the head were not excluded, and were labeled to have mild cervical dystonia (CD) in addition to ET. In these subjects posturing of the head was not a presenting symptom and was found only on careful examination. Detailed neurological examination was also performed. Descriptive statistics were performed using SPSS, version 21.
Results
Overall, 234 subjects were studied. Details of clinical and demographic variables in patients with ET are provided in Table 1 The mean age was 46.6±16.6 years (range 16-86 years), mean AAO was 38.7±17.8 years and mean duration of symptoms was 7.3±7.9 years. Men (n=158) outnumbered women (n=76). Family history of tremor was present in 44.9% (n=105). Upper limb tremor was observed in all (97.5% bilateral, 2.5% unilateral), whereas 38.4% (n=90) patients had lower limb tremor (98.7% bilateral, 1.3% unilateral). Other sites observed to have tremor: voice in 48.7% (n=114), head in 44.4% (n=104), tongue in 6.4% (n=15), and trunk in 0.9% (n=2). Mild CD was observed in 13.7% (n=32). The overlap of clinical features in patients with ET is shown in Figure 1

Figure 1 Venn diagram showing the extent of overlap of head tremor (HT), voice tremor (VT), and mild cervical dystonia (CD) in patients with essential tremor (ET).
Table 1 Clinical and demographic features of patients with essential tremor
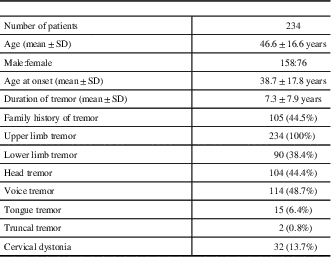
SD: standard deviation.
In the HT+ subgroup (n=104), HT was a presenting symptom (either noted by the patients themselves or a collateral historian before consultation) in 65.4% (n=68). Among the latter, 58.8% (n=40) had HT as the first manifestation of ET, which was later, followed by limb tremor. Hence, nearly one third (34.6%, n=36) of the patients in the HT+ group had asymptomatic head tremor that was observed during the clinical examination at the time of first consultation.
Details of decade-wise analysis of AAO are provided in Figure 2. Two peaks for AAO were observed, one in the second and the other in the fifth decade. On subgroup analysis, the HT+ group had only one peak in the fifth decade, in contrast to the HT− group, which had two peaks like the overall group.

Figure 2 Distribution of patients according to the decade of tremor onset. ET: essential tremor, HT+: essential tremor patients with head tremor; HT−: ET patients without head tremor; HT (initial): ET patients with initial manifestation of head tremor.
Comparison of the HT+ and HT− groups are provided in Table 2. The mean age and AAO in the HT+ group was significantly higher than the HT− group. There was no significant difference between the mean duration of tremor in both groups. The ratio of men to women in the HT+ group was 1.2:1, whereas in the HT− group it was 3.5:1, suggesting a high proportion of women in the HT+ group than the HT− group (p<0.01). In patients with HT as the initial symptom, women outnumbered men (ratio, 2.3:1). When grouped, based on the decade of onset, women outnumbered men in the fifth and sixth decades in the HT+ group, whereas men outnumbered women in rest of the decades. Performing the same analysis on patients with HT as the initial symptom, women outnumbered men in all decades. Details of the decade-wise gender distribution of age at tremor onset are given in Supplementary Table 1.
Table 2 Comparison of ET patients with and without HT
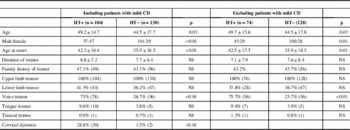
ET: essential tremor; HT: head tremor; HT+: ET patients with head tremor; HT−: ET patients without head tremor; CD: cervical dystonia; NS: not significant.
Age, age at onset, and duration of tremor are presented in years (mean±standard deviation). Number of subjects is in parentheses.
Voice tremor (VT) (75% vs 27.7%, p<0.01) and mild CD (28.8% vs 1.5%, p<0.01) were significantly more common in HT+ patients. The HT+ and HT− groups did not differ from each other significantly on the basis of other clinical features or family history. AAO was earlier in patients with mild CD compared with those without (41.7±3.6 vs 42.5±3.1 years, p=0.02). In the HT+ subgroup, the percentage of patients in which the head was the first affected body part was higher in those with CD compared with those without CD (50% vs 37%, p=0.03).
Comparison Between HT+ and HT− After Excluding Patients with Mild CD
The aim of this analysis was to explore whether the differences discussed previously between the HT+ and HT− groups were influenced by the presence of CD. The parameters, which were significantly different between the HT+ and HT− groups noted previously, remained significant even after excluding the patients with mild CD from the analysis (Table 2). The only change observed was a change in the peak decade of tremor onset in patients with HT to the sixth decade from the previously observed peak at fifth decade.
Discussion
In comparison to previously published studies, our patients were younger and had earlier tremor AAO (mean age in years, mean AAO in years of previous studies; Whaley et alReference Whaley, Putzke, Baba, Wszolek and Uitti 13 : 71±13, 52±22; Louis et alReference Louis and Ottman 14 : 68.9±14.7, 47.1±22.1; Louis et alReference Louis and Dogu 15 : 66.4±16.1, 45.1±21.7). AAO is subject to recall bias that can be minimized by obtaining collateral history from family members,Reference Louis and Ottman 14 which we undertook in our patients. Prior studies have reported a bimodal distribution for age of onset in ET with peaks in the second and sixth decadesReference Louis and Dogu 15 , Reference Lou and Jankovic 16 and inconsistent results regarding gender distribution.Reference Whaley, Putzke, Baba, Wszolek and Uitti 13 , Reference Benito-León, Bermejo-Pareja, Morales, Vega and Molina 17 In our study, we found a bimodal distribution of AAO with peaks in the second and fifth decades, and men outnumbered women. The reason for a skewed gender distribution in our study is not clear, but may partly be due to a referral bias secondary to sociocultural factors: women in our country have less access to medical facilities.
HT has been reported in 35% to 50% of patients with ET in previous clinic and community based studies.Reference Whaley, Putzke, Baba, Wszolek and Uitti 13 , Reference Louis, Ford and Frucht 18 , Reference Hardesty, Maraganore, Matsumoto and Louis 19 We found a similar frequency of HT, despite having a slightly different cohort in terms of age and AAO. In one study, the frequency of HT varied based on the population being studied, being lower in a community setting and higher in a tertiary referral centre (12.3% vs 37.2%).Reference Louis, Pellegrino and Rios 20 This may be attributed to difficulty concealing head tremor from friends and relatives, which would encourage consultation with a specialist. Interestingly, quite often patients with ET are unaware of their HT, even if it was moderate or severe in intensity.Reference Louis, Pellegrino and Rios 20 Lack of self-awareness in HT was also seen in our study: 34.6% of patients with HT did not report it on presentation. This observation is of relevance: studies in which HT is identified based purely on self-reporting are likely to be inaccurate—an important example of this would be genetic linkage studies that are dependent on whether individuals participating are affected or not.
Patients with HT were older and had later onset of tremor as compared with patients without HT. Moreover, patients with HT had a unimodal distribution for AAO with a peak observed in the fifth decade and an almost even gender distribution. No significant differences were observed in duration of tremor between the two groups. These findings are similar to observations published by another group,Reference Louis 9 but they did not report on the distribution of AAO and found that patients with HT were more likely to be women. The authors postulated that development of HT in ET is driven by biological factors intrinsic to the patient, such as age and gender rather than disease-related factors, such as duration of illness.Reference Louis 9 Other studies also suggest that HT is more common in women.Reference Louis, Ford and Frucht 18 , Reference Hardesty, Maraganore, Matsumoto and Louis 19 Although we found HT to be almost equally present in both genders, this was against the trend for the overall ET group wherein men markedly outnumbered women. Moreover, in patients with HT and AAO in the fifth or sixth decade, women outnumbered men, and in all decades, women were more likely than men to have HT as the first symptom. These observations suggest an underlying link between HT and female gender, especially in those patients with HT as the first symptom, and those with onset of symptoms in the fifth and sixth decades.
Previous studies in ET have suggested that HT usually appears after limb tremor.Reference Louis, Ford and Frucht 18 , Reference Hardesty, Maraganore, Matsumoto and Louis 19 In our study, 38.5% of patients had HT as the initial manifestation. Hence, although HT usually follows limb tremor in ET, the reverse does occur. On cross-sectional examination, subjects whose illness begins with HT may present with isolated HT. According to the consensus statement of the Movement Disorder Society, these patients can be classified as having ET, provided they do not have abnormal posturing, which is an exclusion criteria.Reference Deuschl, Bain and Brin 21 A questionnaire study by Pal et al has shown that HT may precede CD.Reference Pal, Samii, Schulzer, Mak and Tsui 22 Can patients with isolated HT and no abnormal posturing go on to develop CD? This is a question that, to our knowledge, remains unanswered. Studies have shown that patients with limb tremor that otherwise looks like classical ET can go on to develop CD.Reference Schiebler, Schmidt and Zittel 23 Some authors suggest that limb tremor in CD is more likely to be irregular, asymmetric, and associated with myoclonus. Electrophysiology may help in further characterization and can help differentiate limb tremor of ET and CD.Reference Shaikh, Jinnah and Tripp 24 , Reference Münchau, Schrag and Rothwell 25 In our study, mild CD was observed in 13.7% of patients with ET and was seen more often in patients with HT. Abnormal posturing is an exclusion criterion for the diagnosis of ET according to the consensus statement of the Movement Disorder Society.Reference Deuschl, Bain and Brin 21 Some authors have suggested that the clinical significance of subtle head or limb posturing in ET is unclear. The exclusion of patients with these findings from research studies may result in too narrow a definition of ET, thereby limiting our ability to identify epidemiological associations. We included patients with mild CD to explore the relationship between HT and mild CD in patients with tremor that otherwise appears consistent with ET. By doing so, we found an association between HT and mild CD; moreover, patients whose onset was with HT were more likely to have mild CD. As the presence of mild CD along with HT may be confused with dystonic HT, we did a comparative analysis of ET patients with and without HT after excluding all the patients with mild CD. However, the findings were not different from the earlier analysis which included patients with mild CD. Because the results remained unchanged even after exclusion of a substantial number patients with mild CD (n=32, 13.7% of the total cohort), the difference between the HT+ and HT− groups observed earlier were unlikely because of the presence of HT secondary to CD.
The prevalence of VT in ET has been reported to be 10% to 62%.Reference Sulica and Louis 26 VT was present in 48.7% of patients in our study, and was more commonly seen in patients with HT. This association could result from head oscillations influencing the vocal air stream and thereby inducing a quiver in the voiceReference Duane 27 or by shared disease-related pathophysiology as outlined in the following section.
The cerebellum has been postulated to be the site of abnormality in ET.Reference Louis 2 The somatotopic organization of the cerebellum is such that head and neck regions are represented medially in the vermis; limbs are represented in the cerebellar hemispheres.Reference Louis, Ford and Frucht 18 The site of initial pathology and its spread could determine the clinical presentation. Based on this hypothesis, patients with cranial tremors (HT and VT) are likely to have pathological changes in the vermis; this has been shown in a study that found a correlation between the number of vermian Purkinje cell torpedoes and cranial tremors.Reference Louis, Faust, Ma, Yu, Cortes and Vonsatell 28 In addition, a magnetic resonance imaging study using voxel-based morphometry has shown vermian atrophy in HT+ patients with ET.Reference Quattrone, Cerasa and Messina 29 In view of the association with older age, normal aging may itself play a role in the manifestation of HT. There is evidence of cerebellar volume loss with normal aging; this loss can preferentially affect certain regions such as the vermis.Reference Bernard and Seidler 30 , Reference Torvik, Torp and Lindboe 31 Gender differences in regional cerebellar volumes have also been described, although these observations have not been consistent.Reference Fan, Tang and Sun 32 The increased frequency of HT in patients who are older and female may be related to the compounding effect of ET pathology on these underlying differences in the cerebellum. Gender differences could also be related to the effect of sex hormones in disease pathogenesis or its clinical presentation.Reference Hardesty, Maraganore, Matsumoto and Louis 19
As discussed, HT+ patients have several distinct clinical and demographic features compared with HT− patients, such as older age, later AAO which has a unimodal distribution, increased association with VT and mild CD, and—although not clearly demonstrated in our study—an association with female gender. In view of these differences, it does appear that ET patients with HT are a distinct clinical phenotype. Being a distinct phenotype, it is currently a matter of speculation whether HT+ patients should be considered a subtype of ET or a separate disease entity. HT in ET and CD have similarities: association with female gender,Reference Duane 27 similar clinical presentation, and slow progression in severity of limb tremor.Reference Münchau, Schrag and Rothwell 25 , Reference Louis, Ford and Barnes 33 This reemphasizes the concern that some patients diagnosed with ET and HT+ have CD as the underlying etiology. This may be especially true for those with HT at onset.
Our study has limitations: because it was a hospital-based study, which evaluated AAO and spread of tremor based on patient history, there is a referral as well as a recall bias in our data. Electrophysiologic assessments were not performed; these could have helped to accurately differentiate ET with HT+ from a dystonic HT in patients with mild CD. The strengths of our study are the large sample size and careful clinical examination of all patients performed by a single movement disorders specialist. Further studies on a larger cohort of ET patients with and without HT along with electrophysiology, voice recording, and vocal cord assessments are warranted to give more insight into our understanding of HT in ET.
DISCLOSURES
None of the authors have any financial disclosures to make or have any conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/cjn.2015.23








