Hemimegalencephaly (HME) is a brain malformation of cortical development characterized by hamartomatous growth of one cerebral hemisphere occasionally with ipsilateral brainstem and cerebellum involvement.Reference Sarnat and Flores-Sarnat1 It is characterized by abnormalities of cellular growth, lineage, differentiation, and migration and, on histology, balloon cells, heterotopias, gliosis, and demyelination are seen.Reference Santos, Escorsi-Rosset and Simao2 Clinically, HME commonly presents with epilepsy, psychomotor delay, contralateral hemiparesis, and hemianopsia.Reference Salamon, Andres and Chute3 MR imaging findings in HME include increased volume of one cerebral hemisphere, abnormal gyration with cortical thickening and polymicrogyria, white matter signal abnormalities, blurring of the gray–white matter interface, abnormally shaped lateral ventricles, and dysmorphic corpus callosum.Reference Re, Scarciolla, Takahashi, Specchio, Bernardi and Longo4 Nevertheless, in some cases, the anatomical boundaries of these abnormalities may be difficult to define with morphologic conventional imaging alone. A good delineation of the lesions is therefore essential, especially if surgery is deemed necessary.
Advanced imaging techniques are producing promising results in the evaluation of HME with white matter fiber tracking as one of the most useful techniques possibly revealing the full extent of white matter tract alterations.Reference Re, Scarciolla, Takahashi, Specchio, Bernardi and Longo4 It has been shown that white matter fiber bundles are not only thicker on the affected side but also that interhemispheric fibers crossing the corpus callosum show variable distribution and asymmetry in a large proportion of these patients.Reference Takahashi, Sato and Ota5 Automated brain segmentation may also be helpful for an accurate quantitative assessment of HME and in the detection of HME in cases where brain asymmetry is not readily apparent. We report a case of HME evaluated with Magnetization Prepared 2-Rapid Acquisition Gradient Echo (MP2RAGE)-based morphometryReference Fujimoto, Polimeni and van der Kouwe6 and diffusion tensor imaging (DTI) to highlight the advantages of using advanced imaging techniques in the workup of this condition.
From an early age, it became apparent that this male patient born to unrelated parents was experiencing asymmetric growth with the left side larger than the right, which was more apparent in lower limb length, and also in the size of the maxilla and the mandible. At the age of 3, he was diagnosed with right oblique astigmatism with his left palpebral fissure larger than the right (9 vs. 6 mm). He also presented deviation of the nose towards the right, which caused altered airflow and breathing difficulties. This resulted in swallowing difficulties, drooling, snoring, and sleep apnea, which were aggravated seasonally by hay fever.
At the age of 12, his facial asymmetry was obvious with the left side larger than the right, made more evident by right eye hypoplasia and enophthalmos with pseudoptosis. There was a considerable gap between the upper and lower dental arches. On examination of the abdomen, there was a surplus of subcutaneous tissue on the right, diastasis of the recti abdomini muscles and a large umbilicus. He presented bilateral skin lesions, more abundant on the left, consistent with cutis marmorata. There was proximal syndactyly on the left between the second and third toes. An abdominal ultrasound scan, also at the age of 12, showed a left kidney larger than the right (92 vs. 86 mm).
At age 16, his left leg was noticeably longer than the right (91 vs. 84 cm) and, since the first signs of puberty were now visible, the decision was made to correct the leg length discrepancy surgically with hemiepiphysiodesis of the left knee.
At age 21, he experienced a first episode of epileptic seizure for which he was referred for a brain MR which showed asymmetry of the cerebral hemispheres, right larger than left, consistent with hemimegalencephaly. Although less prominent, there was also cerebellar asymmetry. The right lateral ventricle was dysmorphic and dilated posteriorly. The gyri were diffusely thickened and small on the right, particularly in the frontal, parietal, and insular regions, compatible with polymicrogyria. The corpus callosum was dysmorphic and larger on the right. White matter T2WI hyperintensity was seen on the right reflecting accelerated myelination and hamartomatous growth. MP2RAGE-based automated segmentation, using the MorphoBox prototype,Reference Fujimoto, Polimeni and van der Kouwe6 confirmed diffuse hypertrophy of brain structures on the right whereas DTI with fiber tracking demonstrated abnormally thickened pathways on the right and, whilst not demonstrating significant asymmetry in the distribution of the interhemispheric fibers crossing the corpus callosum, it did reveal thickened white matter tracts extending more anteriorly on the right frontal lobe and a different orientation of the fibers crossing the splenium of the corpus callosum with a more lateral course on the normal left side (Figure 1).
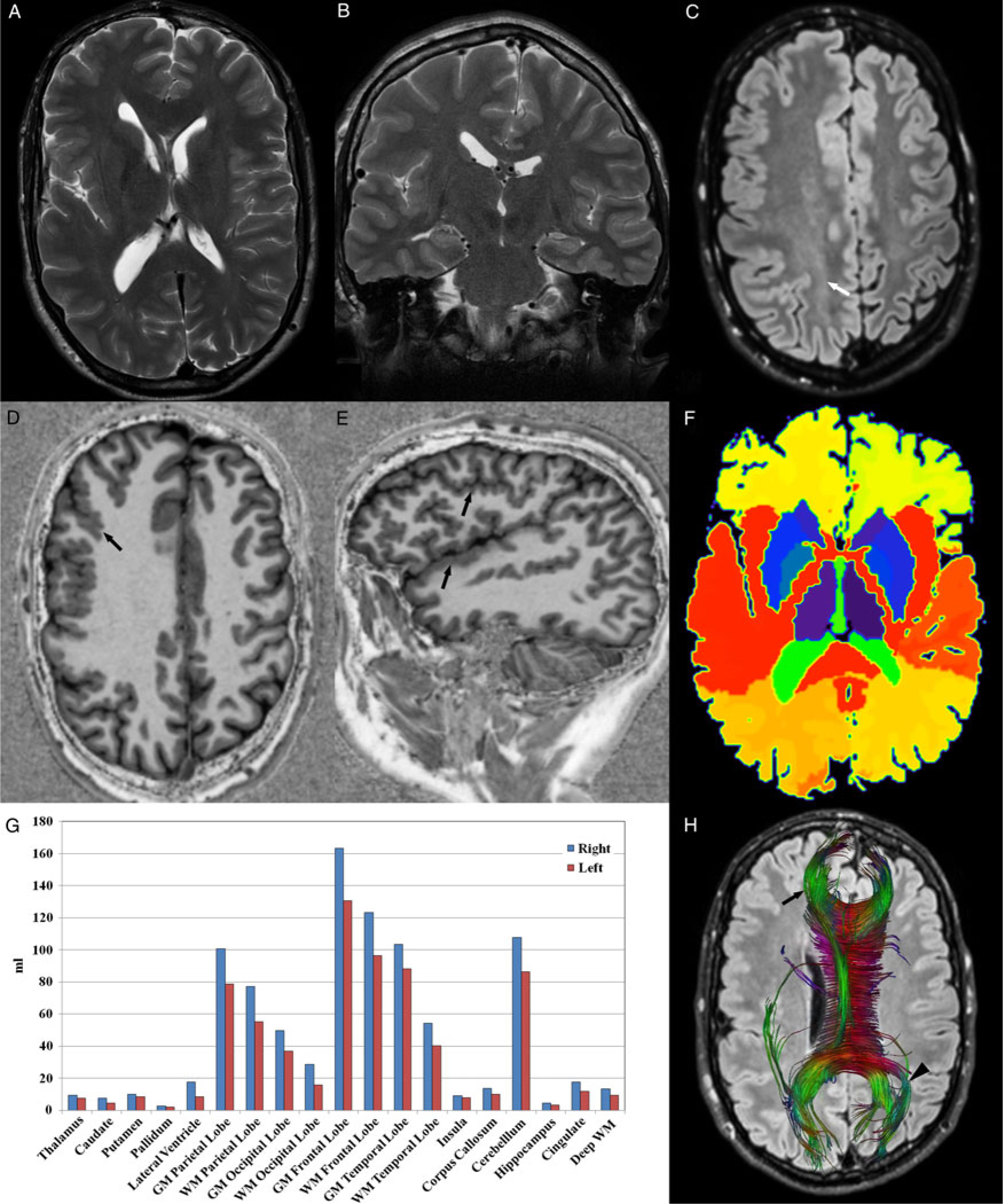
Figure 1: Axial T2WI through the lateral ventricles (A), coronal T2WI through the hippocampi (B), and axial FLAIR through the centrum semi-ovale (C) show brain asymmetry, with the right side larger than the left, and also white matter hyperintensity on the right side, better appreciated on FLAIR (white arrow), reflecting accelerated myelination and hamartomatous growth, typical of hemimegalencephaly. Axial (D) and sagittal (E) MP2RAGE images clearly show abnormalities of cortical development with small thickened gyri on the right frontal, parietal, temporal, and insular regions consistent with polymicrogyria (black arrows). Note the exquisite depiction of the gray–white matter interface, which allows a mask of the different brain structures to be automatically generated (F) and the volume of each structure to be estimated. In this case, all paired brain structures were larger on the right (G), including those where this was not initially apparent such as the hippocampi and the basal ganglia. DTI-based fiber-tracking of the interhemispheric white matter tracks crossing the corpus callosum superimposed on an axial FLAIR image reveal abnormal thickening of white matter tracts crossing the genu of the corpus callosum on the right (black arrow) as well as a more anterior extension of these fibers reflecting the hypertrophy of the right frontal lobe. There is also asymmetry in the orientation of white matter tracts crossing the splenium of the corpus callosum, which display a more lateral course on the normal left side (black arrowhead).
This case illustrates right-sided hemimegalencephaly with contralateral hemihypertrophy, which is less frequently seen but has been previously described.Reference Sharma, Sankhyan, Kabra and Kumar7 Advanced MR imaging techniques clearly demonstrated abnormally thickened white matter tracts with the use of DTI and fiber tracking. Furthermore, MP2RAGE-based automated segmentation allowed accurate estimation of the volume of key brain structures revealing right-sided hypertrophy, even in structures where this was not readily apparent such as the hippocampi and basal ganglia. Additionally, the exquisite depiction of the gray–white matter interface produced by MP2RAGE facilitates the detection of areas of cortical dysplasia and allows T1 mapping of the brain to be generated. This technique could also be used in cases of other developmental abnormalities such as mega corpus callosum as well as in conditions causing regional brain atrophy, especially in children where it is often difficult to delineate these abnormalities.
We believe the use of these advanced sequences could have a place in the imaging workup of HME and in pre-surgical planning as it allows a thorough morphological and quantitative evaluation of the brain as well as analysis of the cerebral microstructure. Furthermore, in cases where the diagnosis of HME is uncertain, this technique is able to reveal asymmetry in brain regions where visual inspection alone might not.
Acknowledgment
The authors wish to thank Mr. Frank Henri for the post-processing of the DTI images and fiber tracking analysis.
Statement of Authorship
JB performed the literature review, drafted the manuscript under the supervision of MIV and selected and edited the images. TK performed the automated brain segmentation and assisted in writing the manuscript. MIV supervised the project, provided expert review of the manuscript, and assisted in selecting the images.
Disclosures
TK is an employee of Siemens Healthcare AG. JB and MIV report no disclosures relevant to the manuscript.





