Zn is an essential nutrient required in man and animals for many physiological functions. Suboptimal intake of dietary Zn is one of the most common nutritional problems worldwide and it has been shown to exist in low-income groups, institutionalized patients, pregnant women and teenagers, as well as in elderly Americans (Walsh et al. Reference Walsh, Sandstead, Prasad, Newberne and Fraker1994). Zn has been shown to be vital as an antioxidant, microtubule stabilizer, anti-apoptotic agent, growth cofactor and anti-inflammatory agent in a variety of tissues (Truong-Tran et al. Reference Truong-Tran, Carter, Ruffin and Zalewski2001). Zn may therefore have important implications for asthma and other inflammatory diseases where the physical barrier is vulnerable and compromised (Truong-Tran et al. Reference Truong-Tran, Carter, Ruffin and Zalewski2001).
It has also been shown that Zn deficiency produces profound alterations in the metabolism of lipids (Cunnane & Yang, Reference Cunnane and Yang1995; Eder & Kirchgessner, Reference Eder and Kirchgessner1995; DiSilvestro & Blostein-Fujii, Reference DiSilvestro and Blostein-Fujii1997; Kettker et al. Reference Kettker, Eder, Kettker and Kirchgessner2000). Nevertheless, reports on the effects of Zn deficiency on lung lipid metabolism are relatively few.
The major phospholipid component (at the air–liquid interface) is phosphatidylcholine (PC) (70–80 %) and de novo PC synthesis from adult lung rats proceeds mainly via the cytidine diphosphocholine (CDP) pathway (Zimmermann et al. Reference Zimmermann, Hogan, Carlson, Smith and Post1993). Dipalmitoylphosphatidylcholine may be synthesized directly via this pathway or by the remodelling of unsaturated PC species (Zimmermann et al. Reference Zimmermann, Hogan, Carlson, Smith and Post1993). The enzyme CTP:phosphocholine cytidylyltransferase (CT) catalyses a rate-regulatory step in the conversion of choline into PC via the Kennedy pathway (Zimmermann et al. Reference Zimmermann, Hogan, Carlson, Smith and Post1993). A previous work showed that a moderate Zn deficiency (in vivo) can induce a physiological stimulus that enhances phospholipid synthesis and changes especially the pattern of phospholipids in adult rat lung (Gomez et al. Reference Gomez, Ojeda and Gimenez2002).
Cholesterol appears to be an integral component of pulmonary surfactant and is second only to dipalmitoylphosphatidylcholine as its major component. Cholesterol enhances the stability of surfactant and controls the fluidity of the gas/liquid monomolecular layer (Hayball & Nicholas, Reference Hayball and Nicholas1989). It may also influence the rate of synthesis of lung phospholipids. Previously, our group found that the increment of cholesterol in serum could be a cause of the increased cholesterol concentration observed in whole lung and macrophages in Zn-deficient animals when compared with the control group (Gomez et al. Reference Gomez, Ojeda and Gimenez2002). On the other hand, it is known that changes in the level of phospholipids would compromise the integrity and function of cell membranes, because these mainly depend on the lipid balance, specifically on the cholesterol:phospholipids ratio (Finney et al. Reference Finney, Nudelman, White, Bursten, Klein, Leer, Wang, Waggoner, Singer and Lewis2000).
In association with these changes, the histopathological study of the lung after Zn deficiency revealed structural damage in lung parenchyma (Gomez et al. Reference Gomez, Ojeda and Gimenez2002).
In the present study, we examine the effect of moderate Zn deficiency on the expression of the rate-regulatory enzymes involved in PC and cholesterol synthesis as the main lipid of lung parenchyma in adult rats.
Materials and methods
Reagents
[3H]H2O (3·70 GBq/g) was purchased from New England Nuclear Life Science Products, Inc. (Boston, MA, USA). Lipid standards were acquired from Sigma Chemical Co. (St. Louis, MO, USA). All the other chemicals were of reagent grade and were obtained from Merck Laboratory (Buenos Aires, Argentina) or from Sigma Chemical Co. Molecular Biology reagents were purchased from Invitrogen (Invitrogen Argentina S.A.) and Promega Inc. (Bio Sciences, USA)
Diet and experimental design
Wistar male rats (200 ± 10 g) were fed two diets with different Zn concentrations. For that purpose, the rats were separated into two groups and fed a Zn-limiting (ZL) diet containing 7 mg Zn/kg and a Zn-adequate control diet supplemented with 30 mg Zn/kg (added as ZnCl2). All the other components of the diet remained constant and were fortified with recommended amounts of vitamins and minerals, according to AIN 93-M diet (Reeves et al. Reference Reeves, Nielsen and Fahey1993). Both diets had the following composition (g/kg): maize starch 466; casein 140 ( ≥ 85 % protein); dextrinized maize starch 155; sucrose 100; fibre/cellulose 50; soyabean oil 40 (containing liposoluble vitamins); mineral mix AIN-93M-MX 35 (Zn was not incorporated into the mineral mix of the ZL diet); vitamin mix 10 (AIN-93-VX); l-cystine 1·8; ascorbic acid 0·008; choline bitartrato 2·5 (41 % choline). All dietary ingredients were monitored for Zn concentration using atomic absorption spectrophotometry. Animals were housed individually in a controlled environment with 12 h light–12 h dark cycle at 21°C. Fresh diets were given and left-over food discarded on a daily basis (20 g diet was enough to ensure ad libitum feeding). Animal treatment protocols were previously approved by the local ethics committee and are in accordance with care and treatment of rats recommended guidelines (US Public Health Service, 1985).
Diet acclimatization lasted for 1 week. During that time, the control diet was provided to all rats and intake was measured. Rats with similar intake profiles during the pre-test were matched and assigned to the control or ZL groups. During the experience, body weights were registered weekly.
Serum and tissue collection
At the end of the experimental period, after 2 months, 12 h after the last feeding, the animals were killed. Rats were anaesthetized intraperitoneally with sodium pentobarbital (50 mg/kg). The respiration of the rat was normal at all times prior to killing (respiratory rate was recorded). Blood for determination of Zn in serum was collected into tubes, washed and rinsed with nitric acid. The lungs were quickly removed, washed with ice-cold 0·9 % saline solution and weighed. Serum and pieces of lobes of each lung were frozen at − 80°C until they were analysed.
Zn analyses
Aliquots of the diet and lung were collected without allowing any contact with metal. Each sample was wet-ashed with 16 N nitric acid as described by Clegg et al. (Reference Clegg, Keen, Lonnerdal and Hurley1981). Zn concentrations of the pre-treated samples and serum were quantified by an atomic absorption spectrophotometer (model 5100, HGA-600 Graphite Furnace; Perkin-Elmer, Argentina S.R.L.). A linear calibration curve using certified standard solutions was carried out. All specimens were diluted by bidistilled, deionized water and analysed in duplicate. Before sample digestion, different amounts of standard solution of each element were added. Recovery was between 98 and 99·2 % for different elements.
Serum lipid determinations
The circulating lipids play a role in the regulation of lipid lung metabolism. We measured serum total cholesterol, HDL-cholesterol and triacylglycerol (TG) by colorimetric or enzymatic methods (kits from Wiener, Buenos Aires, Argentina), using fresh serum. All the determinations were performed within 4 h of obtaining the samples. HDL-cholesterol was measured in the supernatant after precipitation of other lipoproteins by the phosphotungstic-MgCl2 method. LDL-+VLDL-cholesterol was calculated by subtracting the HDL values from total cholesterol values.
Tissue preparation and enzymatic assays
Lung portions (1 g for 4 ml buffer) were homogenized in an Ultra Turrax T25 homogenizer (Rose Sci. Ltd) in 0·5 m-potassium phosphate buffer (pH 7) containing 10 mm-EDTA and 10 mm-d,l-dithiotreitol and protease inhibitors. The homogenates were centrifuged at 100 000 g for 1 h to yield the cytosolic fraction in a Beckman model L8-80M ultracentrifuge (Beckman Coulter Instruments Inc., USA) with a Ty-80 rotor.
Cytosolic fatty acid synthase (FAS) activity was determined spectrophotometrically by a modified version of the method of Alberts et al. (Reference Alberts, Ferguson, Hennessy and Vagelos1974). The reaction mixture contained: 0·5 m-potassium phosphate buffer (pH 6·6); EDTA and dithiotreitol (1 μmol each); 100 nmol NADPH; the cytosolic fraction (0·05 ml). Adding 100 nmol malonyl-CoA started the reaction and the final assay volume was 1·05 ml. The oxidation of NADPH at 30°C was monitored at 340 nm. FAS activity was expressed as units/mg cytosolic proteins.
To measure glucose-6-phosphate dehydrogenase (G6PDH), NADP-isocitrate dehydrogenase and NADP-malic dehydrogenase activity, lungs were homogenized with Tris-HCl buffer, pH 7·4, containing 1 mm-dithiotreitol. The homogenates were centrifuged at 100000 g for 1 h and the enzymatic activities were measured in the supernatant. G6PDH, isocitrate dehydrogenase and malic dehydrogenase were determined by the rate of NADPH formation at 340 nm according to Glock & McLean (Reference Glock and McLean1953)Farrell (Reference Farrell1980) and Bukato et al. (Reference Bukato, Kochan and Swierczynski1995).
Lipid determinations
Lipids were extracted from lung tissue and total lipids were determined by dry weight. The lipids were resuspended in hexane–isopropanol mixture (3:2, v/v), containing butylated hydroxytoluene as antioxidant (Hara & Radin, Reference Hara and Radin1978). Aliquots were taken in order to determine phospholipids and to measure P (Rouser et al. Reference Rouser, Fluster and Yamamoto1970) and total cholesterol (Abell et al. Reference Abell, Levy, Brodie and Kendall1952). Another part of the extracts was used for the separation of the different lipids on TLC plates coated with silica gel G (Merck, Darmstadt, Germany) using hexane–diethyl ether–acetic acid (80:20:1, by vol.) as solvent. The lipids were detected by exposing the plates to I vapours. They were scraped off and used directly for the determination of phospholipids (Rouser et al. Reference Rouser, Fluster and Yamamoto1970), TG (Sardesai & Manning, Reference Sardesai and Manning1968), free and esterified cholesterol (Abell et al. 1952).
Incorporation of [3H] from 3H2O into lung lipids
All animals were fed ad libitum and then injected intraperitoneally with 37 mBq mCi/rat [3H]H2O in 1 ml saline solution. They were killed 2 h later to ensure that newly synthesized lipids in the lung had been labelled and 0·250 g lung was extracted with hexane–isopropanol mixture (3:2, v/v) containing butylated hydroxytoluene as antioxidant (Hara & Radin, Reference Hara and Radin1978). The different lipid fractions were separated by TLC by duplicate (see previous paragraph). They were scraped off and used for determining the incorporated radioactivity in a Beckman LS 100c liquid scintillation counter and the other sample was used for determining the different lipids (Gomez et al. Reference Gomez, Ojeda and Gimenez1999). The results are expressed as ng 3H incorporated/h per g tissue.
RNA isolation and RT-PCR analysis
Using TRIzol (Life Technologies Inc., USA), we isolated total RNA. Gel electrophoresis and ethidium bromide staining confirmed the purity and integrity of the samples. Quantification of RNA was based on spectrophotometric analysis at 260/280 nm.
Total RNA (10 μg) was reverse-transcribed using MMLV Reverse Transcriptase (Promega Inc.) and random hexamers as primers. RT-generated fragments coding for β-actin, acetyl CoA carboxylase, glycerol-3-phosphate acyltransferase, diacylglycerol acyltransferase (Waterman et al. Reference Waterman, Price and Zammit2002), lipoprotein lipase (Zhou et al. Reference Zhou, Wang, Higa, Newgard and Unger1999), sterol regulatory element binding protein (SREBP)-2 and 1c and CT (Carter et al. Reference Carter, Waite, Campenot, Vance and Vance2003) were amplified using PCR.
PCR was performed in reaction solution (35 μl) containing 0·2 mm-dNTP, 1·5 mm-MgCl2, 1·25 U Taq polymerase, each rat specific oligonucleotide primer (50 pmol) and RT products (1/10 of RT reaction). The sequences of the different primers as well as the size of the expected PCR products are shown in Table 1. Samples were heated to 94°C for 2 min, followed by thirty-eight temperature cycles. Each cycle consisted of three periods: (1) denaturation, 94°C for 1 min; (2) annealing, 55°C for lipoprotein lipase, acetyl CoA carboxylase, diacylglycerol acyltransferase, glycerol-3-phosphate acyltransferase, SREBP-2 and 1c and β-actin and 60°C for CT for 1 min; (3) extension, 72°C for 1 min. After thirty-eight reaction cycles, the extension reaction was continued for another 5 min.
Table 1 Sequences of the primers used to amplify different genes by RT-PCR*
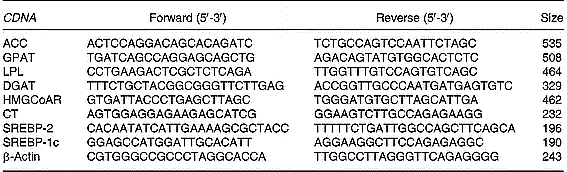
ACC, acetyl CoA carboxylase; GPAT, glycerol-3-phosphate acyltransferase; LPL, lipoprotein lipase; DGAT-1, diacylglycerol acyltransferase 1; HMG-CoAR, 3-hydroxy-3-methylglutaryl CoA reductase; CT, CTP:phosphocholine cytidylyltransferase, SREBP, sterol regulatory element binding protein.
* For details of procedures, see p. 1040.
The PCR products were electrophoresed on 2 % agarose gel with 0·01 % ethidium bromide. The image was visualized and photographed under UV transillumination and the bands were quantified using NIH Image software (Tucows Inc., USA) and reported as the values of band intensity units. The relative abundance of each band was then normalized according to the housekeeping gene β-actin, calculated as the ratio of the intensity values of each product to that of β-actin.
Statistics
Statistical analysis was performed using Student's t test when only two groups were compared (Snedecor & Cochran, Reference Snedecor and Cochran1967). When variances were not homogeneous, we performed log transformation of the data. Differences between means were considered significant at the P < 0·05 level.
Results
Weight gain and Zn status of the rats
Rats fed low Zn were ZL based on body weight and serum Zn concentration (DiSilvestro & Blostein-Fujii, Reference DiSilvestro and Blostein-Fujii1997). Lung weight was significantly lower in ZL after 2 months of treatment (Table 2). There was no change in daily food intake. In our experimental model, clinical signs such as dermatitis or alopecia were not observed.
Table 2 Effects of Zn-limited (ZL) diet on body weight and Zn concentrations in serum and lung after 2 months of treatment† (Values are means with their standard errors for ten rats per group)
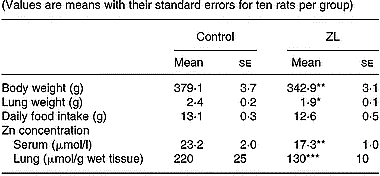
Mean values were significantly different from those for the control group: *P < 0·05; **P < 0·01; ***P < 0·001.
† For details of animals and procedures, see p. 1039.
Total lipids, cholesterol and triacylglycerol in serum
Given the fact that circulating lipids play a role in the regulation of lung lipid metabolism, we measured the concentrations of several lipids and proteins in the serum of control and ZL rats after 2 months of treatment (Table 3). The increase in (LDL+VLDL) cholesterol fraction in the ZL group was responsible for the increase in total cholesterol. There were no changes in the other measures: proteins, total lipids, triacylglycerol and HDL-cholesterol between treatment groups.
Table 3 Effects of Zn-limited (ZL) diet on serum concentrations of proteins and different lipids after 2 months of treatment† (Values are means with their standard errors for eight rats per group)
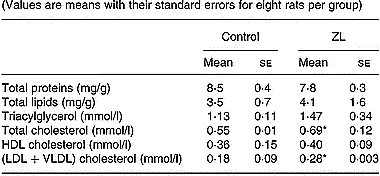
Mean values were significantly different from those for the control group: *P < 0·05.
† For details of animals and procedures, see p. 1039.
Triacylglycerols, phospholipids and cholesterol in whole lung
As shown in Table 4 the concentration of triacylglycerol decreased while phospholipids, free and esterified cholesterol increased when compared with the control group. Under this condition, the concentration of phospholipids and esterified cholesterol was higher in the ZL group.
Table 4 Influence of Zn-limited (ZL) diet on triacylglycerol, free and esterified cholesterol, phospholipid concentrations and activities of fatty acid synthase (FAS) and dehydrogenases in lung after 2 months of treatment† (Values are means with their standard errors for eight rats per group)
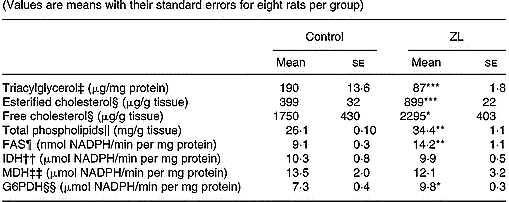
Mean values were significantly different from those for the control group: *P < 0·05; **P < 0·01; ***P < 0·001.
† For details of animals and procedures, see p. 1039.
‡ Determined using the method of Sardesai & Manning (Reference Sardesai and Manning1968).
§ Determined using the method of Abell et al. (1952).
‖ Determined using the method of Rouser et al. (Reference Rouser, Fluster and Yamamoto1970).
¶ Determined using the method of Alberts et al. (Reference Alberts, Ferguson, Hennessy and Vagelos1974).
†† Isocitrate dehydrogenase (IDH) determined using the method of Farrell (Reference Farrell1980).
‡‡ Malic dehydrogenase (MDH) determined using the method of Bukato et al. (Reference Bukato, Kochan and Swierczynski1995).
§§ Glucose-6-phosphate dehydrogenase (G6PDH) determined using the method of Glock & McLean (Reference Glock and McLean1953).
Activity of fatty acid synthase and dehydrogenases enzymes in lung
The activity of the lipogenic enzyme FAS showed variations as well as the activity of G6PDH. The increase of FAS was higher than that of G6PDH. However, no changes were observed in isocitrate dehydrogenase and malic dehydrogenase activities (Table 4).
Incorporation of [3H] from 3H2O into lung lipids
The incorporation of 3H from 3H2O into the different lipids (specific activity) was modified by Zn deficiency. We observed a decrease in the incorporation into TG, whereas there was an increased incorporation into phospholipids and esterified cholesterol (Table 5). There were no changes in the incorporation of 3H into free cholesterol between the treated groups.
Table 5 Effects of Zn-limited (ZL) diet on the incorporation of 3H from [3H]H2O into the lung lipids† (Values are means with their standard errors)
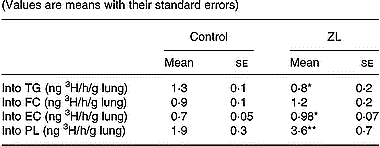
TG, triacylglycerol; FC, free cholesterol; EC, esterified cholesterol; PL, phospholipid.
Mean values were significantly different from those of the respective control groups using Student's t test: *P < 0·05; **P < 0·01.
† For details of procedures, see p. 1040.
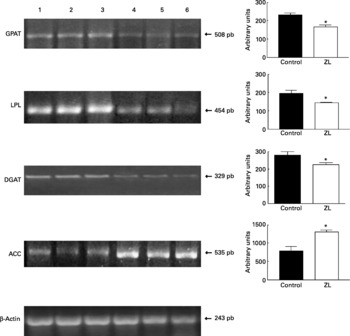
Expression of glycerol-3-phosphate acyltransferase, lipoprotein lipase, diacylglycerol acyltransferase and acetyl CoA carboxylase in lung
Fig. 1 shows that the expression of glycerol-3-phosphate acyltransferase, lipoprotein lipase and diacylglycerol acyltransferase mRNA was decreased in the ZL group and in concordance with the enzymatic activity of FAS, the expression of acetyl CoA carboxylase mRNA was increased in the ZL rats.
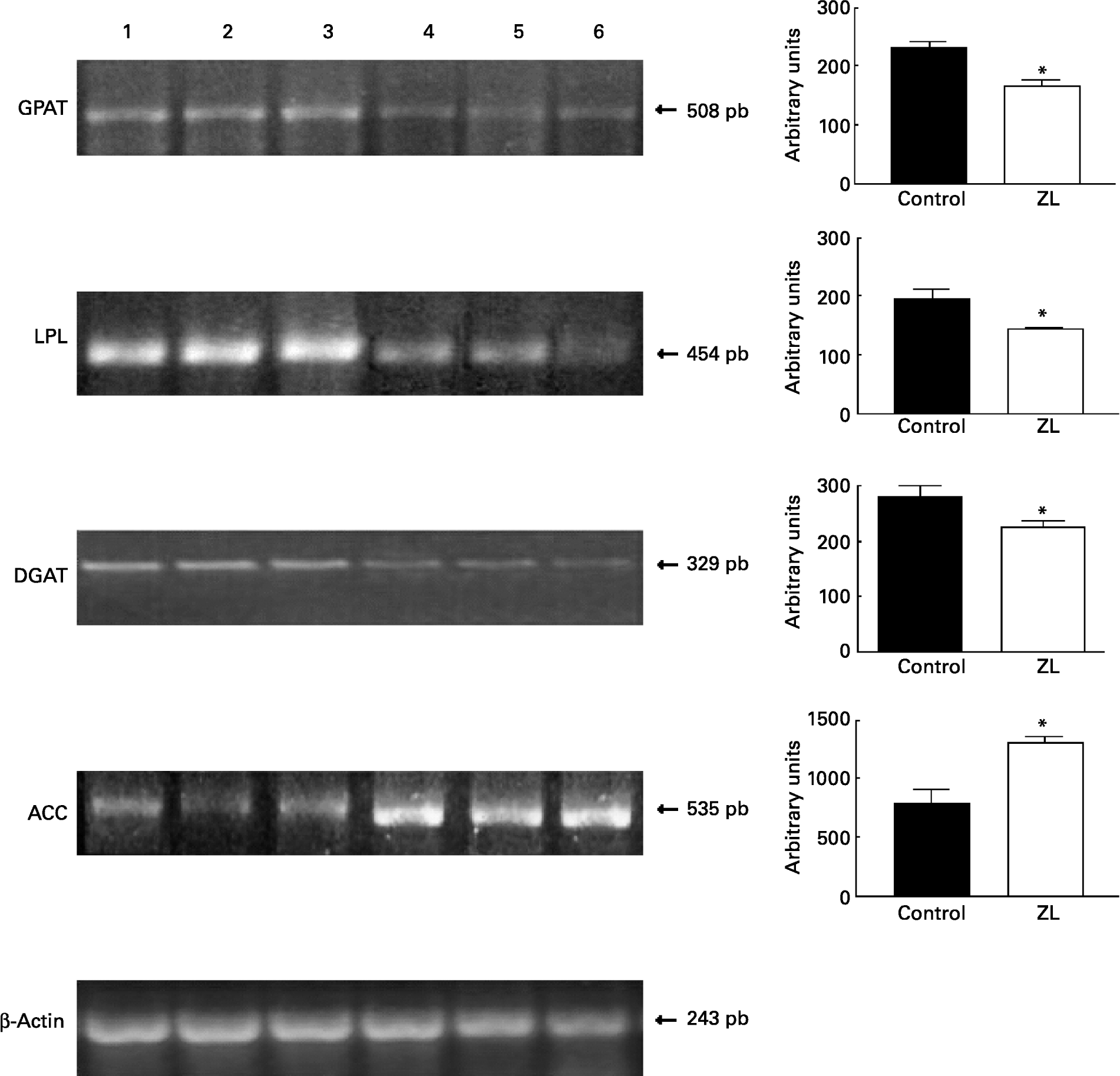
Fig. 1 Expression of enzymes of the triacylglycerol pathway in control and Zn-deficient rats together with the quantification of the intensity of the fragment bands in relation to the intensity of the internal control bands. Ethidium bromide-stained agarose gel of glycerol-3-phosphate acyltransferase (GPAT), lipoprotein lipase (LPL), diacyl glycerol acyltransferase (DGAT), acetyl CoA carboxylase (ACC) and β-actin PCR products used as an internal control. Lanes 1–3, control samples; lanes 4–6, Zn limited (ZL) samples; pb, pair of bases. Values were significantly different from those of controls; *P < 0·05. For details of animals and procedures, see p. 1040.
Expression of 3-hydroxy-3methylglutaryl CoA reductase and sterol regulatory element binding protein-2
We evaluated the expression of 3-hydroxy-3-methylglutaryl CoA reductase (HMGCoAR), the rate-limiting enzyme of cholesterol synthesis. Fig. 2 shows that HMGCoAR decreased its expression. Fig. 3 shows that there were no changes in the SREBP expression between treated groups.
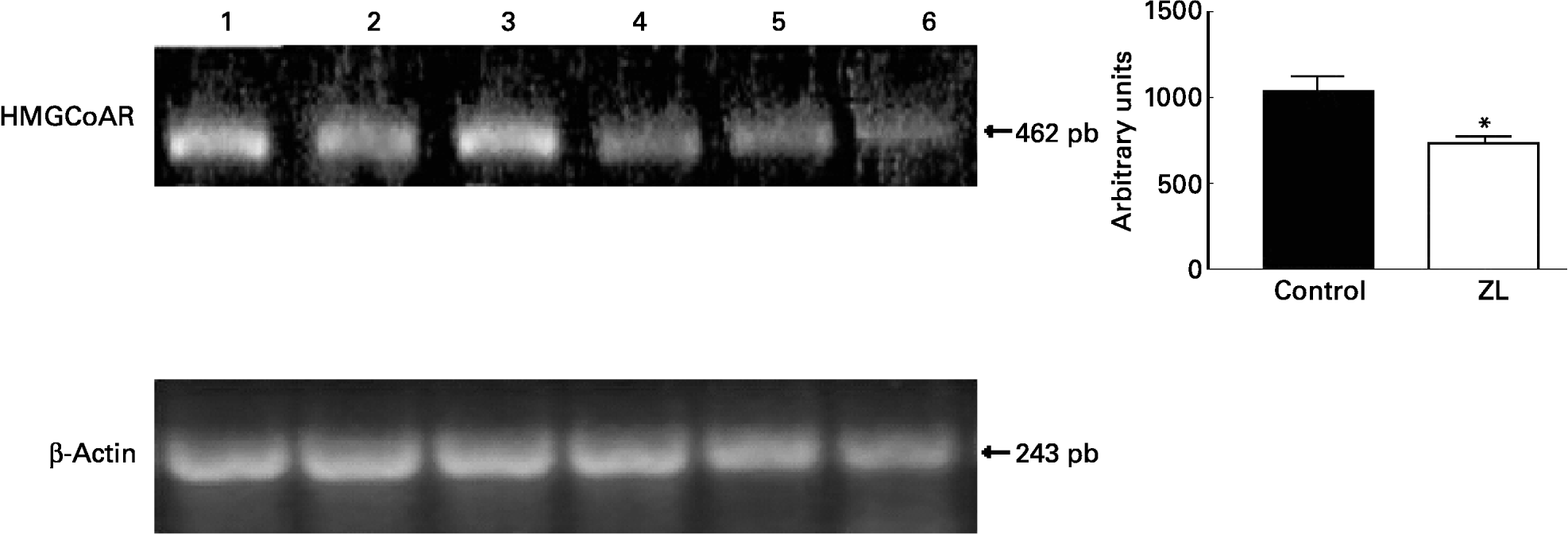
Fig. 2 Expression of genes involved in the cholesterol synthetic pathway together with the quantification of the intensity of the fragment bands in relation to the intensity of the internal control bands. Ethidium bromide-stained agarose gel of 3-hydroxy-3-methylglutaryl CoA reductase (HMGCoAR) and β-actin PCR products used as an internal control. Lanes 1–3, control samples; lanes 4–6, Zn limited (ZL) samples; pb, pair of bases. Value was significantly different from that of controls; *P < 0·01. For details of animals and procedures, see p. 1040.

Fig. 3 Expression of sterol regulatory element binding protein (SREBP)-2 and 1c together with the quantification of the intensity of the fragment bands in relation to the intensity of the internal control bands. Ethidium bromide-stained agarose gel of SREBP-2, SREBP-1c and β-actin PCR products used as an internal control. M, molecular weight marker; pb, pair of bases; lanes 1–3, control samples; lanes 4–6, Zn limited (ZL) samples. For details of animals and procedures, see p. 1040.
Expression of CTP:phosphocholine cytidylyltransferase
We then tried to determine if the observed increase of phospholipids was due to an increment of the content of PC by studying the expression of CT, the main regulator of that biosynthetic pathway (Fig. 4), which was effectively increased.

Fig. 4 Expression of CTP:phosphocholine cytidylyltransferase (CT) together with the quantification of the intensity of the fragment bands in relation to the intensity of the internal control bands. Ethidium bromide-stained agarose gel of CT and β-actin PCR products used as an internal control. M, molecular weight marker; pb, pair of bases; lanes 1–3, control samples; lanes 4–6, Zn limited (ZL) samples. Value was significantly different from that of controls; **P < 0·005. For details of animals and procedures, see p. 1040.
Discussion
It is known that Zn deficiency influences the lipid metabolism in a variety of ways (Eder & Kirchgessner, Reference Eder and Kirchgessner1995; Shiratori et al. Reference Shiratori, Houweling, Zha and Tabas1995; Kettker et al. Reference Kettker, Eder, Kettker and Kirchgessner2000). However, little is known about changes in lung lipid metabolism, specifically in phospholipid content, due to Zn deficiency. A study from our laboratory showed that lipid concentration in the lung (mainly phospholipids) and the content of PC are modified by a moderate Zn deficiency (Gomez et al. Reference Gomez, Ojeda and Gimenez2002). For this reason, the aim of the present study was to elucidate which of the lung enzymes involved in lipid metabolism are affected by a ZL diet. For this purpose, a rat model that allows the induction of a ZL diet was used (Shiratori et al. Reference Shiratori, Houweling, Zha and Tabas1995; Gomez et al. Reference Gomez, Fernandez, Zirulnik, Ojeda and Gimenez2003; Pfaffl et al. Reference Pfaffl, Gerstmayer, Bosio and Windisch2003). This model has the advantage that it can be used for a long time and it is physiologically more relevant (Cunnane & Yang, Reference Cunnane and Yang1995; Shiratori et al. Reference Shiratori, Houweling, Zha and Tabas1995; Gomez et al. Reference Gomez, Fernandez, Zirulnik, Ojeda and Gimenez2003, Reference Gomez, Davicino, Biaggio, Bianco, Alvarez, Fischer, Masnatta, Rabinovich and Gimenez2006; Pfaffl et al. Reference Pfaffl, Gerstmayer, Bosio and Windisch2003). This model is more relevant because severe Zn deficiency can impair many physiological processes, including protein synthesis and feed intake. Also, marginal intake of Zn is common in industrialized and developing countries. Consequently, marginal Zn deficiency is believed to be more prevalent than once thought (Christian & West, Reference Christian and West1998).
In our experimental design, the decreased body weight observed in ZL rats confirmed previous results (Cunnane & Yang, Reference Cunnane and Yang1995; Shiratori et al. Reference Shiratori, Houweling, Zha and Tabas1995; DiSilvestro & Blostein-Fujii, Reference DiSilvestro and Blostein-Fujii1997). The degree of Zn deficiency was evidenced by the reduction of Zn concentrations in lung and serum (Shiratori et al. Reference Shiratori, Houweling, Zha and Tabas1995; DiSilvestro & Blostein-Fujii, Reference DiSilvestro and Blostein-Fujii1997). The animals exposed to a ZL diet showed an increase in the levels of total cholesterol and VLDL+LDL; this situation leads to increased vulnerability to oxidative injury (DiSilvestro & Blostein-Fujii, Reference DiSilvestro and Blostein-Fujii1997). In accordance with this situation, our laboratory found that moderate Zn deficiency in rats could be associated with nitrosative and oxidative stress in lung (Gomez et al. Reference Gomez, Davicino, Biaggio, Bianco, Alvarez, Fischer, Masnatta, Rabinovich and Gimenez2006).
Fatty acid synthesis is an important metabolic pathway that is under complex hormonal and nutritional control. In the present study, we found a decrease in lung TG synthesis evidenced by the diminished incorporation of 3H[H2O]. We also studied the activity of FAS and we found it increased by 36 % when compared with control; the expression of acetyl CoA carboxylase also showed a significant increase (Fig. 1). These observations suggest that the amount of fatty acid production may be increased. Those results were also supported by the changed activity of a dehydrogenase enzyme (G6PDH) that provides NADPH for the activity of FAS among other uses such as the regeneration of reduced glutathione. Moreover, only G6PDH increased its activity by 23 %, while malic dehydrogenase and isocitrate dehydrogenase did not change in the ZL group (Table 4). Perhaps, the level of reactive oxygen species could affect the levels of NADPH, given the fact that Tavazzi et al. (Reference Tavazzi, Di Pierro, Amorini, Fazzina, Galvano, Lupi, Giardina and Lazzarino1999) determined that the pyridine coenzymes could be considered target molecules of reactive oxygen species. This situation was confirmed by our group, which provided experimental evidence of the pro-oxidant effects of a moderate Zn deficiency in adult rat lung (Gomez et al. Reference Gomez, Fernandez, Zirulnik, Ojeda and Gimenez2003, Reference Gomez, Davicino, Biaggio, Bianco, Alvarez, Fischer, Masnatta, Rabinovich and Gimenez2006).
Lipoprotein lipase, an enzyme linked to hydrolysis and uptake of TG from circulation, was determined in order to study specifically the contribution of exogenous fatty acids in ZL lung. ZL produced a decrease in the expression of lipoprotein lipase when compared with the control group. The present results show that there is not an external contribution of fatty acids in this organ through this way. We would suggest that the fatty acids involved in the synthesis of phospholipids and esterified cholesterol in adult rat lung are increased specifically by endogenous synthesis. Thus, physiological control of these synthetic processes is often coordinated at both transcriptional and post-translational levels. It is known that phospholipids and TG are metabolically interconnected by common lipid intermediates, such as diacylglycerol and fatty acids, which are exchanged in a recycling pathway that could be potentially relevant for membrane synthesis and lipid signal transduction (Igal et al. Reference Igal, Caviglia, de Gomez Dumm and Coleman2001; Bagnato & Igal, Reference Bagnato and Igal2003). Moreover, enzymes for both pathways are subjected to common transcriptional regulation. However, little is known about the mechanism of potential co-regulation of TG and phospholipid metabolism (Caviglia et al. Reference Caviglia, De Gomez Dumm, Coleman and Igal2004). Recently, Caviglia et al. provided evidence indicating that TG synthetic enzymes such as mitochondrial glycerol-3-phosphate acyltransferase may indirectly regulate phospholipid synthesis by controlling the availability of lipid substrates (mainly fatty acids and diacylglycerol) for phospholipid formation (Caviglia et al. Reference Caviglia, De Gomez Dumm, Coleman and Igal2004). On the other hand, a change in tissue fatty acid composition depending on Zn status has been reported for a variety of tissues (Ayala & Brenner, Reference Ayala and Brenner1983; Cunnane et al. Reference Cunnane, Horrobin and Manku1984) with major differences in the concentrations of oleic, linoleic and arachidonic acid (Ayala & Brenner, Reference Ayala and Brenner1983; Cunnane et al. Reference Cunnane, Horrobin and Manku1984; tom Dieck et al. 2004) and in the ratio of saturated fatty acids and MUFA:PUFA (Eder & Kirchgessner, Reference Eder and Kirchgessner1993; tom Dieck et al. Reference tom Dieck, Doring, Fuchs, Roth and Daniel2004). However, changes of activities of Δ5-, Δ6-, Δ9-desaturases were not consistently found in Zn-deficient rats (Eder & Kirchgessner, Reference Eder and Kirchgessner1994, Reference Eder and Kirchgessner1995). tom Dieck et al. (Reference tom Dieck, Doring, Fuchs, Roth and Daniel2004) provide evidence for a change in fatty acid composition of hepatic lipids in Zn-deficient rats. This situation most likely reflects the down regulation of enzymes required for fatty acid oxidation in peroxisomes and mitochondria with specificity for SCFA. On the other hand, the palmitate uptake of type II cells increased in parallel with an increase of cholesterol-ester content (Kolleck et al. Reference Kolleck, Gutyhmann, Ladhoff, Tandon, Schlame and Rustow2002). Under these conditions, a small but significant increase of palmitate incorporation into phospholipids occurred. For this reason, in this experimental model there might be a redistribution of substrates, where the synthesis of PC is prioritized.
As in our model the amount of 1-acyl-sn-glycerol-3-phosphate decreased, we could suggest that PC would be increased as a consequence of a decreased TG synthesis. We also determined diacylglycerol acyltransferase expression that decreased significantly, which suggests that there would be less synthesis of TG.
Regarding the metabolism of cholesterol, we found an increase in the content of total cholesterol, as a consequence of increased free and esterified cholesterol fractions. However, esterified cholesterol increased more than free cholesterol; this could be due to a higher availability of endogenous fatty acids for cholesterol esterification, perhaps as: (a) a consequence of increased fatty acid synthesis; (b) an increase in cholesterol esterase activity; (c) influx of exogenous fatty acids or a combination of some of those factors. In addition, Hayball & Nicholas (Reference Hayball and Nicholas1989) found that exogenous cholesterol is rapidly esterified in the alveolus. In the present study, higher concentrations of cholesterol fractions are correlated with the incorporation of 3H into esterified cholesterol fractions and a slight increment of 3H incorporated into free cholesterol. To elucidate aspects of this relationship, we measured the expression of HMGCoAR, the rate-limiting enzyme in the pathway, which decreased significantly, perhaps as a consequence of the high level of lung cholesterol. The present results are coincident with those presented by Panini et al. (Reference Panini, Schnitzer-Polokoff, Spencer and Sinensky1989). It is known that the expression of HMGCoAR is regulated by SREBP-2. But when we measured the expression of this factor, we did not find significant differences when compared with control. On the other hand, it is known that the activation of SREBP-2 depends on two sequential cleavages. The second cleavage requires the action of a Zn metalloprotease (Brown & Goldstein, Reference Brown and Goldstein1999). In the present experimental conditions, SREBP-2 activation might be diminished due to the absence of Zn. Consequently, we think that the diminution in the expression of HMGCoAR in lung could be due to different factors: as higher influx of cholesterol; less activation of SREBP-2 by Zn deficiency. This would lead to a decreased expression of HMGCoAR.
Regarding phospholipids, we have found that phospholipid contents were increased in the Zn-deficient model. In the present study, we analysed the incorporation of (3H (-H2O into phospholipids, which was also increased. As PC is the main component of the lung surfactant, and its production was found to be increased (Gomez et al. Reference Gomez, Ojeda and Gimenez2002), we measured the expression of CT and found that it was also increased. We observed that cholesterol increased in the ZL model, a situation that led to an increased expression and activity of CT (Shiratori et al. Reference Shiratori, Houweling, Zha and Tabas1995; Gomez et al. Reference Gomez, Ojeda and Gimenez2002).
Modifications in the major lipid pathways lead specifically to physiological changes. In the present experimental model, the changes observed in lipid pathways reflect a compensatory response to ensure the availability of substrates for phospholipid and cholesterol biosynthetic pathways.
An increasing number of reports are emerging, indicating that changes in PC metabolism are a common feature of the pathophysiology of experimental chronic lung injury or adult respiratory distress syndrome (Vivekananda et al. Reference Vivekananda, Smith and King2001). Lipid oxidation products have recently been implicated in end-organ damage in human sepsis, including lung injury; these findings suggest important interactions between cytokines, neutrophils, lipids (specifically oxidized forms) and oxygen radicals in the development of acute inflammatory lung injury.
In conclusion, the present results suggest that moderate Zn deficiency produces alterations in the expression of the enzymes involved in phospholipids, specifically PC, and cholesterol synthesis in lung, which lead to increased phospholipids and cholesterol while TG decreases. This situation is accompanied by an important oxidative and nitrosative stress associated with significant morphological changes in lung parenchyma (Gomez et al. Reference Gomez, Fernandez, Zirulnik, Ojeda and Gimenez2003, Reference Gomez, Davicino, Biaggio, Bianco, Alvarez, Fischer, Masnatta, Rabinovich and Gimenez2006). In addition, Frey et al. (Reference Frey, Haupt, Alms, Holzmann, Konig, Kern, Kox, Rustow and Schlame2000) demonstrated that fragmented phospholipids increase in response to various forms of oxidative stress. On the other hand, fragmented phospholipids were reported in patients with adult respiratory distress syndrome, in which both lung inflammation and hyperoxia are common (Frey et al. Reference Frey, Haupt, Alms, Holzmann, Konig, Kern, Kox, Rustow and Schlame2000). Perhaps, when this situation occurs together with other different pathologies in lungs, it could lead to the worst prognosis in patients at risk.
It would be important to apply the knowledge of Zn deficiency as a stimulus to understand the complexities of the changes produced in lung lipid metabolism. This situation contributes to a better understanding of the pathophysiology and clinical significance of Zn deficiency in order to design health interventions in lung.
Acknowledgements
This research was supported by a grant from CONICET-4931 and National University of San Luis, Project No. 8104. The authors would like to thank Dr. A. Acosta, Miss I. Sosa and, Mr. R. Dominguez, for their technical assistance.