Obesity is prevalent in modern society due to a lifestyle consisting of the high consumption of dietary fat and sucrose and little exercise( Reference Vadiveloo, Scott and Quatromoni 1 , Reference Siervo, Montagnese and Mathers 2 ). The population of adults with a BMI of 25 kg/m2 or greater has increased globally( Reference Ng, Fleming and Robinson 3 ). Obesity is associated with a state of chronic low-grade inflammation, which is a risk factor for insulin resistance and links to obesity-associated diseases such as CVD, type 2 diabetes and cancer( Reference Lumeng and Saltiel 4 – Reference Morris, Hudis and Giri 7 ). Adipose tissue plays a critical role in energy homoeostasis due to its function as lipid storage and endocrine organ. As an endocrine organ, adipose tissue secretes adipocytokines, which regulate feeding, thermogenesis, immunity and neuroendocrine function( Reference Ahima 8 ).
Non-alcoholic fatty liver disease (NAFLD), which is defined by excessive hepatic TAG content in the absence of excessive alcohol consumption, is prevalent in obese subjects and is a potential risk factor for the development of both type 2 diabetes and metabolic syndrome( Reference Yki-Järvinen 9 – Reference Marchesini, Brizi and Bianchi 12 ). The quantifiable biological sources of hepatic TAG were directly detected in NAFLD patients by Donnelly et al.( Reference Donnelly, Smith and Schwarzenberg 13 ): 59·0 % of TAG arose from NEFA, which flow to the liver via the lipolysis pathway in adipose tissue; 26·1 % from de novo lipogenesis; and 14·9 % from dietary fat. Sterol regulatory element-binding protein-1c (SREBP-1c) is a transcription factor that stimulates the expression of genes related to de novo lipogenesis( Reference Foretz, Pacot and Dugail 14 , Reference Horton, Bashmakov and Shimomura 15 ). Under high-fat (HF) diet feeding, the levels of mRNA and protein of SREBP-1c and TAG increase in the liver( Reference Ai, Zhu and Min 16 ). PPARγ2 is another nuclear receptor involved in lipid metabolism and a nutrient sensor in metabolic tissues( Reference Vidal-Puig, Jimenez-Liñan and Lowell 17 ). The expression of Pparγ2 is increased in response to an HF diet, and it leads to the development of NAFLD( Reference Yamazaki, Shiraishi and Kishimoto 18 ).
Soyabeans provide one of the most abundant plant sources of dietary protein. The protein content of soyabeans varies from 36 to 56 %, and this diversity is due to the areas in which the soyabeans are grown( Reference Grieshop and Fahey 19 , Reference Garcia, Torre and Marina 20 ). A meta-analysis of human studies indicates that the consumption of soya protein is associated with significant decreases in serum TAG and cholesterol( Reference Anderson, Johnstone and Cook-Newell 21 ). Moreover, soya protein is reported to be effective in lowering body weight (BW) and fat mass in overweight and obese subjects( Reference Allison, Gadbury and Schwartz 22 , Reference Velasquez and Bhathena 23 ).
In all, 80 % of soyabean protein is constituted by glycinin (11S globulin) and β-conglycinin (7S globulin)( Reference Garcia, Torre and Marina 20 ). The content of β-conglycinin, the second most present protein in whole soya protein, is reported to be about 30 %( Reference Iwabuchi and Yamauchi 24 ). Administration of β-conglycinin to rats prevented increases in serum total cholesterol (TC), TAG and VLDL-TAG( Reference Ferreira, Silva and Demonte 25 – Reference Tachibana, Iwaoka and Hirotsuka 27 ). Moreover, in a human randomised, double-blind, placebo-controlled study, 12-week consumption of 5 g of β-conglycinin per day significantly reduced serum TAG concentrations in subjects with hypertriacylglycerolaemia, whereas consumption of 5 g of casein did not( Reference Kohno, Hirotsuka and Kito 28 ). Thus, β-conglycinin has effects on the prevention and improvement of hyperlipidaemia. Therefore, it is likely that β-conglycinin might prevent and improve not only hyperlipidaemia but also NAFLD and obesity. We previously reported that BW gain and NAFLD in mice induced by an HF diet were prevented by the simultaneous supplementation of β-conglycinin for 11 weeks through the down-regulation of liver Pparγ2 and its target gene expression( Reference Yamazaki, Kishimoto and Miura 29 ). Hence, β-conglycinin seems to have beneficial effects on the amelioration of NAFLD by down-regulating hepatic Pparγ2 expression. However, there is no report on the effects of β-conglycinin on the improvement of NAFLD and obesity.
Given this background, in this study, we investigated the effects of dietary β-conglycinin on the improvement of NAFLD and obesity in HF diet-induced obese (DIO) mice and clarified the mechanism underlying these effects in liver and white adipose tissue (WAT).
Methods
Animals
Male ddY mice, 6 weeks old, a model of postprandial hypertriacylglycerolaemia in response to dietary fat( Reference Yamazaki, Kishimoto and Ezaki 30 ), were obtained from Japan SLC, Inc. and fed a normal laboratory diet (CE2; Clea) for 1 week to stabilise their metabolic condition. Mice were exposed to a 12 h light–12 h dark cycle, and the room was maintained at a constant temperature of 22°C. They were individually housed and allowed free access to experimental diets and water. Mice were cared for in accordance with the National Institutes of Health’s (NIH) Guide for the Care and Use of Laboratory Animals. All animal procedures were reviewed and approved by the National Institute of Health and Nutrition, Japan (no. 1507).
Dietary experiments
To examine the effect of β-conglycinin on the improvement of fatty liver and obesity, ddY mice at 7 weeks of age were fed an HF diet (60 energy% fat) for 4 weeks to generate diet-induced obesity. Thereafter, these DIO mice were assigned to one of six groups (n 6): the HF, medium-fat (MF) and low-fat (LF) groups, in which the mice were fed a 60, 30 and 10 energy% fat diet, respectively, supplemented with casein, and in three other HF, MF and LF groups, all casein was replaced by β-conglycinin. The HF diet is too high in fat and is slightly difficult for humans to ingest ordinarily, so we created the MF dietary group to investigate this fat level, which humans ordinarily ingest, and to examine the effect of β-conglycinin. Detailed compositions of the experimental diets are listed in Table 1. Duration of the dietary manipulations was 5 weeks in this experiment. Diets were prepared as mentioned in our previous study( Reference Yamazaki, Kishimoto and Miura 29 ). Butter was purchased from Snow Brand Milk Corp.. Safflower oil was purchased from Benibana Food. β-Conglycinin, prepared by treatment of soyabean protein extract with phytase( Reference Saito, Kohno and Tsumura 31 ), was kindly provided by Fuji Oil Co. The purity of β-conglycinin determined by SDS-PAGE was more than 95 %. Consumption of food was measured daily. Food intake per day was estimated by subtracting the food weight of that day from the initial food weight of the previous day. Average energy intakes during total experimental periods in each group of mice were calculated with these data.
Table 1 Dietary composition of the experimental diets
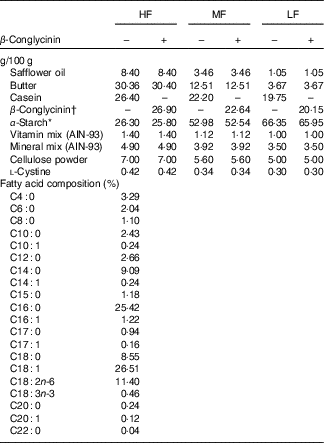
HF, high fat; MF, medium fat; LF, low fat.
* Pregelatinised maize starch.
†β-Conglycinin contains 5% carbohydrate (as glucose) and 95% protein. The fatty acid composition is the same in all diets.
Measurement of VO2 and carbon dioxide production
Open-circuit indirect calorimetry was performed with an O2/CO2 metabolism measuring system for small animals (MK-5000RQ; Muromachi Kikai Co., Ltd) 1 week before execution as described previously( Reference Yamazaki, Kishimoto and Miura 29 ). The system monitored VO2 and carbon dioxide production (VCO2) at 3-min intervals and calculated the RQ ratio (VCO2:VO2). Spontaneous motor activity was measured using a Supermex infrared sensor (Muromachi Kikai Co., Ltd). Measurements were performed for the dark (from 19.00 to 07.00 hours) and light (from 07.00 to 16.30 hours) periods under ad libitum feeding conditions. The energy production rate was calculated with the formulae used by Ferrannini in which the rate of energy production (kJ/min (kcal/min))=3·91 VO2+1·10 VCO2−3·34 N, glucose oxidation (g/min)=4·55 VCO2–3·21 VO2 and lipid oxidation (g/min)=1·67 (VO2−VCO2), where N is the rate of urinary nitrogen excretion used to estimate protein oxidation( Reference Ferrannini 32 ). However, considering that only a small portion of the resting and exercise energy expenditure arises from protein oxidation, the contributions of protein oxidation were neglected. These measurements were only done on the HF mice.
Quantitative RT PCR
Mice were killed by cervical dislocation, and livers and epididymal and subcutaneous (posterior subcutaneous depots) WAT were isolated for RNA preparation in the morning from 3-h fasted animals to avoid acute effects of food intake. RNA was extracted with TRIzol Reagent (Invitrogen Corp.) according to the manufacturer’s instructions. Isolated RNA was quantified by using a NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific), and its integrity was confirmed by agarose gel electrophoresis. Total RNA isolated from tissues was reverse transcribed with ReverTra Ace (Toyobo Co., Ltd) with random hexamers. The resulting complementary DNA was PCR amplified in the ninety-six-well format with SYBR Green PCR Master Mix and a 7500 Real-Time PCR System (Applied Biosystems). Expression levels of test genes were normalised to those of an endogenous control, acidic ribosomal phosphoprotein P0 (36B4). The primers used for quantitative real-time PCR are listed in the online Supplementary Table S1.
Serum chemistries
Serum glucose was measured on an Ascensia autoanalyzer (Bayer Medical, Ltd). Serum TAG, TC and NEFA levels were respectively assayed by enzymatic colorimetry with TAG E, TC E and NEFA C test kits (Wako Pure Chemical Industries, Ltd). Serum insulin, leptin and adiponectin were determined with a mouse insulin ELISA kit (Morinaga), a mouse leptin ELISA kit (Morinaga) and a mouse adiponectin ELISA kit (Otsuka Pharmaceutical Co.), respectively.
Western blot analysis
Liver nuclear protein was extracted with a Nuclear Extract Kit (Active Motif) according to the manufacturer’s instructions. Protein (100 μg) separated by SDS-PAGE (7·5 % gel) was electrophoretically transferred onto Clear Blot Membrane-P (ATTO) and detected with specific primary antibodies: PPARγ2 (PA1-824, 1:1000 dilution; Thermo Scientific) or β-actin (C4) (sc47778, 1:5000 dilution; Santa Cruz Biotechnology, Inc.). Peroxidase-conjugated anti-rabbit or mouse IgG (1:8000 dilution; Santa Cruz Biotechnology) was used as the secondary antibody. Bands were visualised with an enhanced chemiluminescence system (GE Healthcare) and quantified with NIH Image software (NIH).
Liver and faecal lipid analysis
Liver lipids were measured by enzymatic colorimetry as described previously( Reference Yamazaki, Kishimoto and Miura 29 ). Lipids in the liver were extracted quantitatively with ice-cold 2:1 (v/v) chloroform–methanol by the method of Folch et al. ( Reference Folch, Lees and Sloane Stanley 33 ). TAG and TC concentrations in the liver were respectively measured by enzymatic colorimetric methods using the TC E and TAG E tests (Wako Pure Chemicals, Ltd). Faeces were collected for 1 d 1 week before execution and dried in an FD-1000 freeze dryer (Tokyo Rikakikai Co., Ltd) for 24 h. The trap cooling temperature was −45°C. After drying, faeces lipid was extracted by chloroform–methanol (2:1)-acetic acid (4 %) by the method of Folch et al. ( Reference Folch, Lees and Sloane Stanley 33 ). Faeces TAG and TC were measured with the TAG E and TC E test kits described above.
Statistical analysis
Values are shown as means with their standard errors. Two-way ANOVA was used to examine the two main effects of dietary fat and β-conglycinin and their interaction (IBM SPSS Statistics 23). When we found a significant interaction, we performed a Test of Simple Effects with SPSS using the Estimated Marginal Means option. If there was no statistically significant interaction, but there was a statistically significant difference in mean interest, we performed a post hoc test for the different levels of fat. Statistical significance was set at P<0·05.
Results
β-Conglycinin supplementation ameliorates obesity of diet-induced obese mice
To investigate the effect of dietary β-conglycinin on the amelioration of obesity, mice were fed an HF diet for 4 weeks to generate DIO mice. The average initial BW of the DIO mice was 47·8 (se 0·5) g (n 36). Then, the DIO mice were grouped into the HF, MF and LF groups. Further, casein or β-conglycinin was given as a dietary protein to the DIO mice. After 5 weeks of feeding of the experimental diets, although there was no difference in total energy intake, BW and relative weight of subcutaneous WAT were greater with higher fat in the diet, and regardless of the dietary fat level, β-conglycinin reduced these weights. There was a significant main effect of dietary fat for relative epididymal, retroperitoneal and mesenteric WAT (all higher with greater dietary fat level) but no effect of β-conglycinin for consumption (Table 2).
Table 2 Body weight (BW), relative tissue weights and total energy intake of mice after 5 weeks on experimental diets(Mean values with their standard errors and two-way ANOVA P values, n 6)
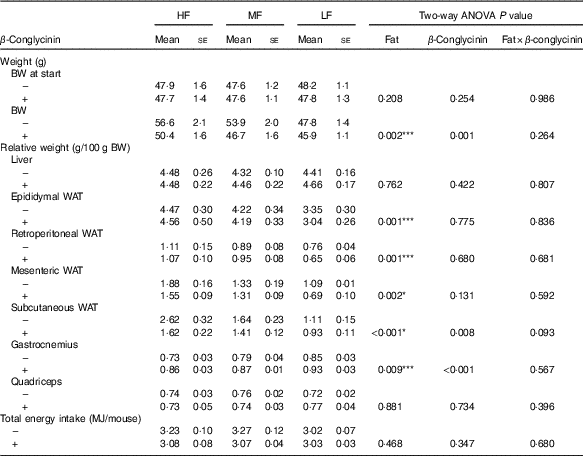
HF, high fat; MF, medium fat; LF, low fat; WAT, white adipose tissue.
* P<0·05, HF different from MF and LF; *** P<0·05, HF different from LF.
Effects of β-conglycinin on blood glucose, serum lipid and serum adipocytokines in diet-induced obese mice
After 5 weeks of the experimental diets, the concentration of leptin was greater with higher fat in the diet, and regardless of the dietary fat level, the β-conglycinin-supplemented mice had a significantly lower leptin concentration (Table 3). Furthermore, the insulin concentration was drastically lower in the β-conglycinin-fed group than in the casein-fed group. However, β-conglycinin had no effect on the concentrations of serum glucose, TAG, TC, NEFA and adiponectin.
Table 3 Serum chemistries(Mean values with their standard errors and two-way ANOVA P values, n 6)
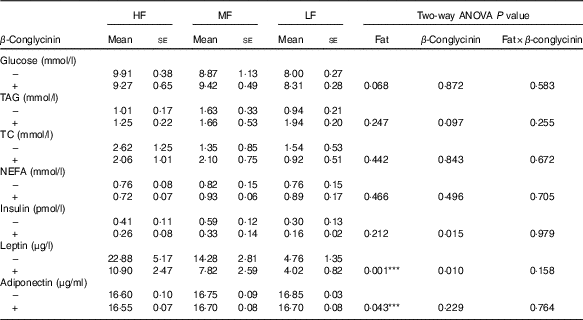
HF, high fat; LF, low fat; MF, medium fat; TC, total cholesterol.
*** P<0·05, HF different from LF.
β-Conglycinin ameliorates liver TAG accumulation in diet-induced obese mice
Fatty liver was generated after 4 weeks of the HF diet( Reference Yamazaki, Shiraishi and Kishimoto 18 ). After 5 further weeks of feeding with the experimental diets, liver TAG accumulation was greater with higher fat in the diet, and regardless of the dietary fat level, the β-conglycinin-supplemented mice had significantly lower TAG accumulation (Fig. 1(a)). Compared with the casein group fed the same level of fat, β-conglycinin decreased the hepatic TAG concentration by 34·8 % in the HF dietary group, 43·0 % in the MF dietary group and 34·6 % in the LF dietary group. However, there was no significant difference in liver TC concentration among the groups (Fig. 1(b)).
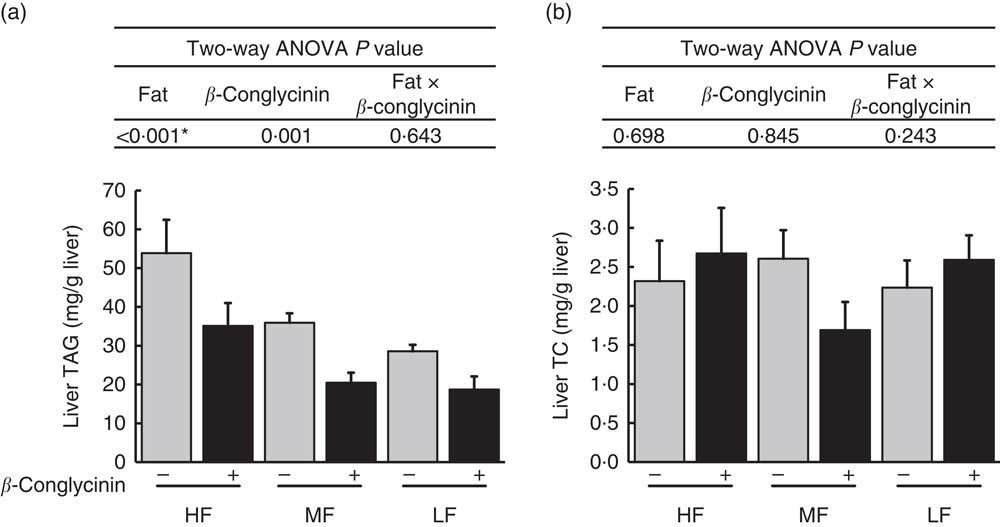
Fig. 1 Hepatic lipids concentrations (mg/g liver): TAG concentrations (a), TC concentration (b). Values are means (n 6), with their standard errors represented by vertical bars. HF, high fat; MF, medium fat; LF, low fat; TC, total cholesterol; ![]() , concentrations in mice fed a casein diet;
, concentrations in mice fed a casein diet; ![]() , concentrations in mice fed a β-conglycinin diet. Two-way ANOVA P values are significant in regard to effects of diet. * P<0·05, HF different from MF and LF.
, concentrations in mice fed a β-conglycinin diet. Two-way ANOVA P values are significant in regard to effects of diet. * P<0·05, HF different from MF and LF.
Effects of β-conglycinin on hepatic gene expression and nuclear protein concentration
To elucidate the mechanisms underlying the effect of β-conglycinin on the improvement of fatty liver, hepatic gene expression was examined by quantitative real-time PCR. The mRNA expression of PPARγ2 rose in the β-conglycinin-unsupplemented HF diet-fed mice, and β-conglycinin significantly lowered this expression (Fig. 2(a)). Moreover, fatty acid translocase (CD36), one of the target genes of Pparγ2, which markedly rose in response to the HF diets, was also affected by β-conglycinin supplementation (Fig. 2(a)). The amount of nuclear PPARγ2 protein was also significantly lower in the β-conglycinin-supplemented HF diet-fed mice than in the β-conglycinin-unsupplemented HF diet-fed mice (Fig. 2(b)).
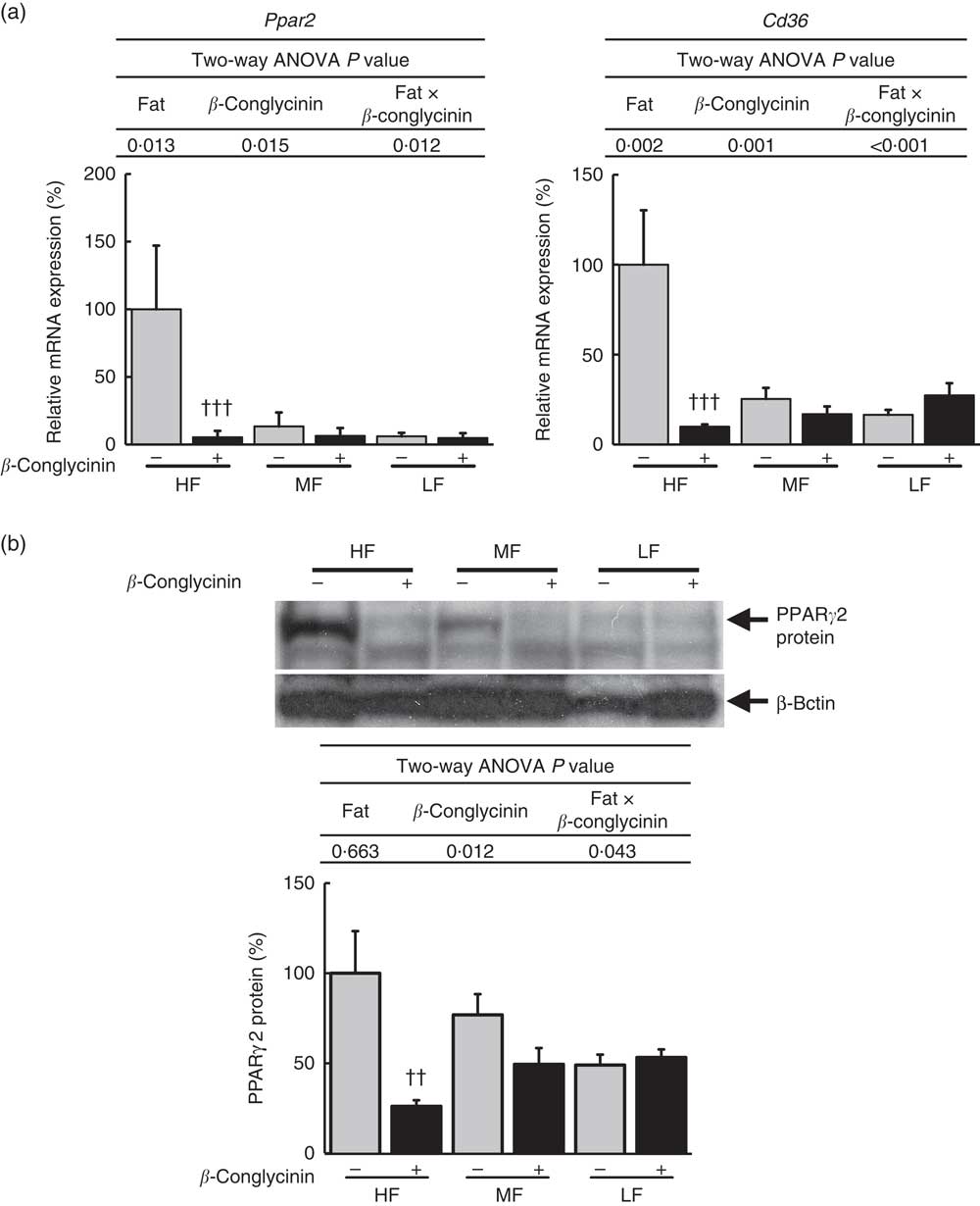
Fig. 2 Hepatic Pparγ2 gene and protein and target gene expressions. Pparγ2 and its target gene expression (a), PPARγ2 protein level (b). Values are means (n 6), with their standard errors represented by vertical bars. HF, high fat; MF, medium fat; LF, low fat; ![]() , concentrations in mice fed a casein diet;
, concentrations in mice fed a casein diet; ![]() , concentrations in mice fed a β-conglycinin diet. Two-way ANOVA P values are significant in regard to effects of diet and interaction. †† P<0·01, ††† P<0·001, β-conglycinin different from control (casein).
, concentrations in mice fed a β-conglycinin diet. Two-way ANOVA P values are significant in regard to effects of diet and interaction. †† P<0·01, ††† P<0·001, β-conglycinin different from control (casein).
β-Conglycinin significantly suppressed the mRNA expressions of SREBP-1c, a transcriptional factor by which de novo lipogenesis is stimulated, and its target genes, such as fatty acid synthase (FAS), stearoyl-CoA desaturase 1 (SCD1), and acetyl-CoA carboxylase 1 (ACC1) (Fig. 3(a)). PPARα, responsible for fatty acid oxidation, and its target gene, medium-chain acyl-CoA dehydrogenase, were not affected by β-conglycinin supplementation (Fig. 3(b)). These data indicate that the suppression of Pparγ2 and Srebp-1c and their target genes may contribute to the mechanisms causing the improvement of fatty liver induced by an HF diet.
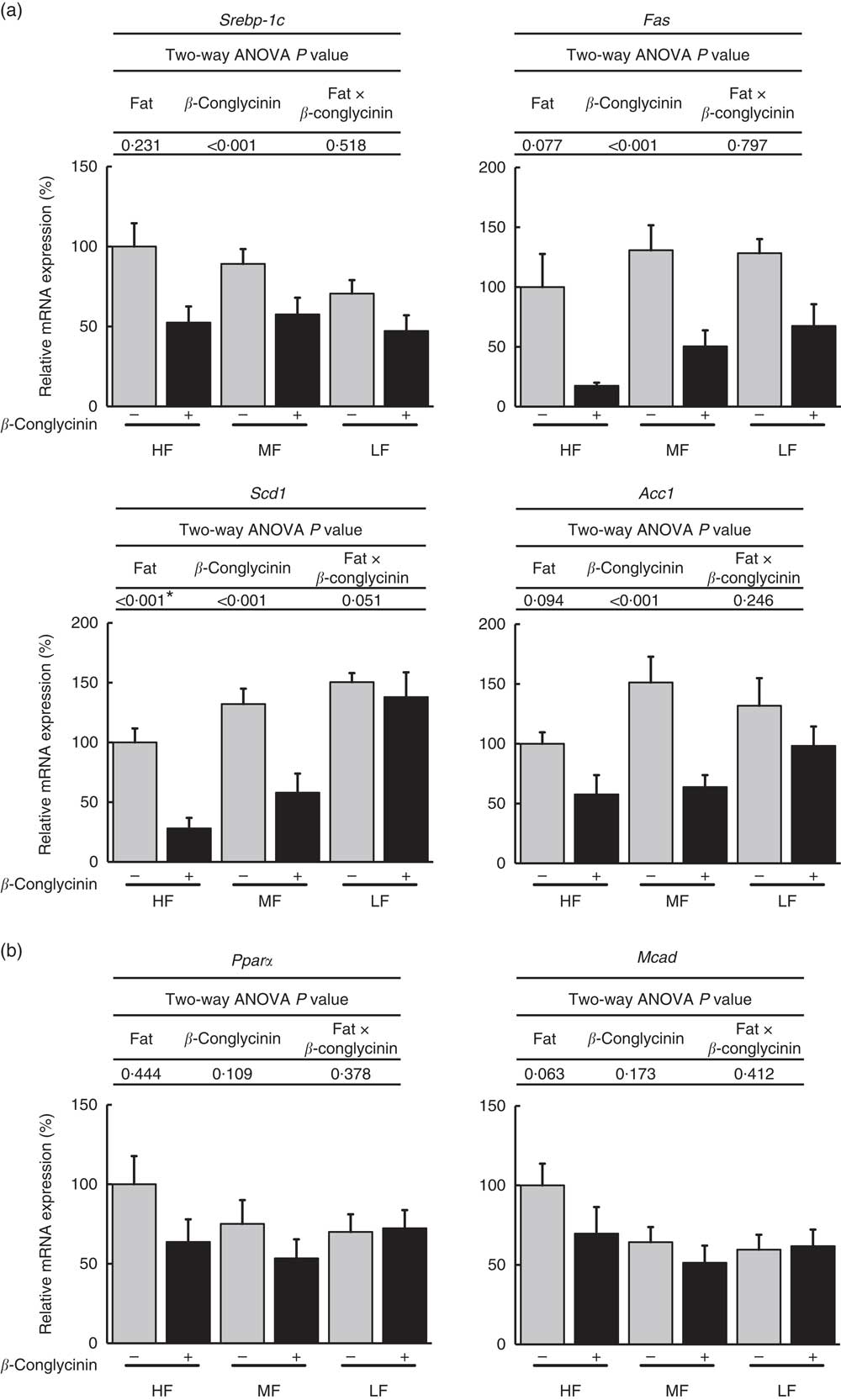
Fig. 3 Hepatic gene expression related to lipid metabolism. Sterol regulatory element-binding protein-1c (Srebp-1c) and its target genes (a), Pparα and its target gene (b). Values are means (n 6), with their standard errors represented by vertical bars. HF, high fat; MF, medium fat; LF, low fat; Fas, fatty acid synthase; Scd1, stearoyl-CoA desaturase-1; Acc1, acetyl-CoA carboxylase 1; Mcad, medium-chain acyl-CoA dehydrogenase; ![]() , concentrations in mice fed a casein diet;
, concentrations in mice fed a casein diet; ![]() , concentrations in mice fed a β-conglycinin diet. Two-way ANOVA P values are significant in regard to effects of diet. * P<0·05, HF different from MF and LF.
, concentrations in mice fed a β-conglycinin diet. Two-way ANOVA P values are significant in regard to effects of diet. * P<0·05, HF different from MF and LF.
Effects of β-conglycinin on gene expressions in epididymal and subcutaneous white adipose tissue
Dietary supplementation of β-conglycinin ameliorated obesity induced by HF diet feeding. To elucidate the molecular mechanism behind the effect of dietary β-conglycinin on lipid metabolism and inflammation in adipose tissue, the mRNA levels of macrophage markers, adipocytokine genes and lipid metabolism-related genes were measured in epididymal and subcutaneous WAT. In epididymal WAT, β-conglycinin significantly suppressed the mRNA expression of macrophage markers CD68 and F4/80 in the HF diet-fed mice and monocyte chemotactic protein-1 (MCP-1) (Fig. 4(a)). β-Conglycinin supplementation changed the mRNA levels of leptin in the MF diet-fed mice but did not cause a change in that of adiponectin. β-Conglycinin supplementation did not change the expression of lipid metabolism-related genes Pparγ1, Pparγ2 and fatty acid binding protein (aP2) in epididymal WAT (Fig. 4(b) and (c)).
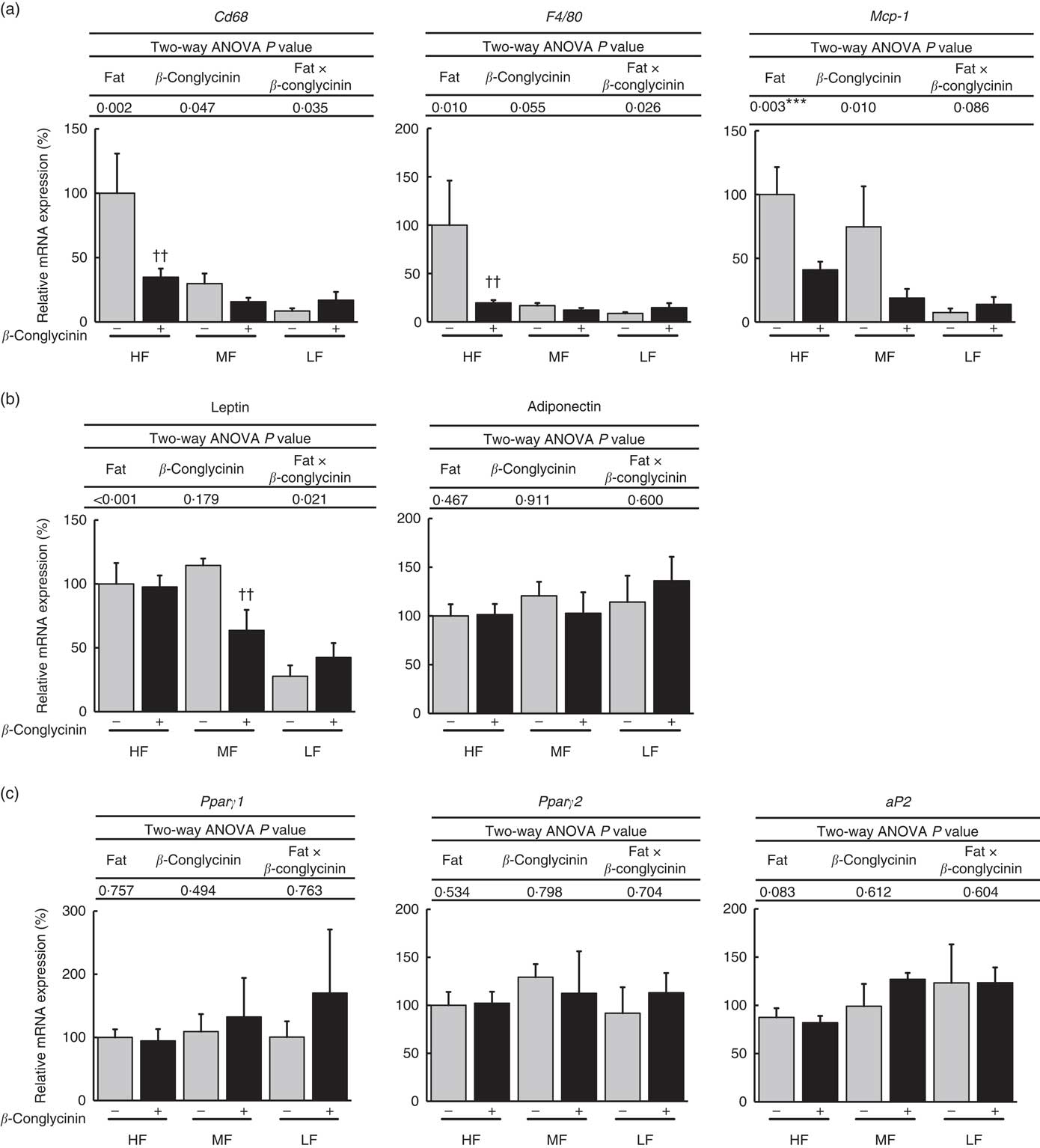
Fig. 4 Epididymal white adipose tissue (WAT) gene expression. Inflammation-related genes (a), adipocytokine genes (b), Pparγ1/2 and their target gene (c). Values are means (n 6), with their standard errors represented by vertical bars. HF, high fat; MF, medium fat; LF, low fat; Mcp-1, monocyte chemotactic protein-1; aP2, fatty acid binding protein; ![]() , concentrations in mice fed a casein diet;
, concentrations in mice fed a casein diet; ![]() , concentrations in mice fed a β-conglycinin diet. Two-way ANOVA P values are significant in regard to effects of diet and interaction. *** P<0·05, HF different from LF; †† P<0·01, β-conglycinin different from control (casein).
, concentrations in mice fed a β-conglycinin diet. Two-way ANOVA P values are significant in regard to effects of diet and interaction. *** P<0·05, HF different from LF; †† P<0·01, β-conglycinin different from control (casein).
As for subcutaneous WAT, the mRNA expression of macrophage markers CD68, F4/80 and MCP-1 was suppressed by the dietary supplementation of β-conglycinin in the HF dietary group (Fig. 5(a)). Moreover, β-conglycinin supplementation dramatically suppressed the mRNA levels of adipocytokines, leptin and adiponectin in subcutaneous WAT in the HF dietary group (Fig. 5(b)). β-Conglycinin significantly suppressed the expression of lipid metabolism-related genes Pparγ1 in the HF dietary group and Pparγ2 in the HF and MF dietary groups. β-Conglycinin also significantly suppressed the expression of aP2 (Fig. 5(c)).
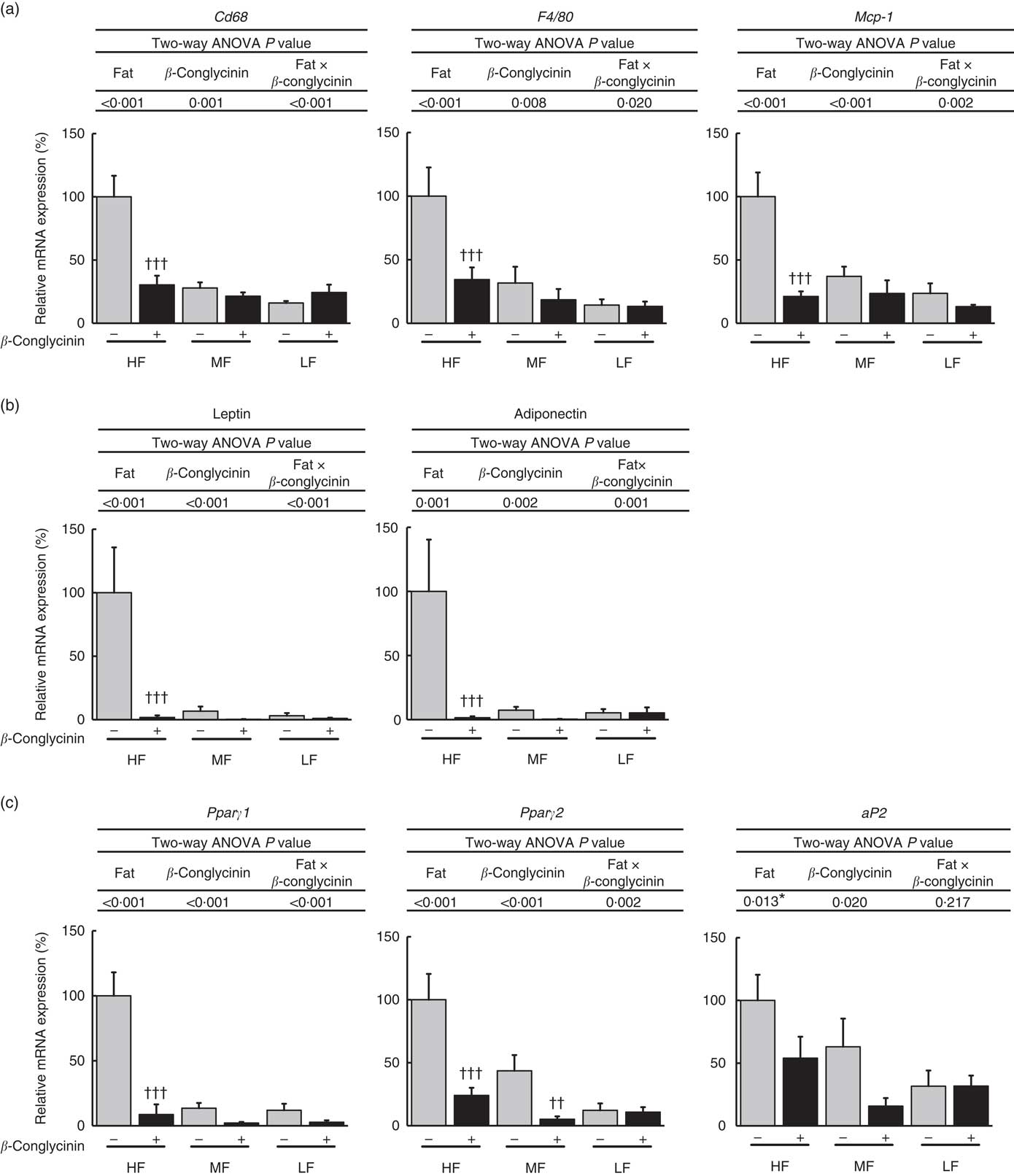
Fig. 5 Subcutaneous white adipose tissue (WAT) gene expression. Inflammation-related genes (a), adipocytokine genes (b), Pparγ1/2 and their target gene (c). Values are means (n 6), with their standard errors represented by vertical bars. HF, high fat; MF, medium fat; LF, low fat; Mcp-1, monocyte chemotactic protein-1; aP2, fatty acid binding protein; ![]() , concentrations in mice fed a casein diet;
, concentrations in mice fed a casein diet; ![]() , concentrations in mice fed a β-conglycinin diet. Two-way ANOVA P values are significant in regard to effects of diet and interaction. * P<0·05, HF different from MF and LF; †† P<0·01, ††† P<0·001, β-conglycinin different from control (casein).
, concentrations in mice fed a β-conglycinin diet. Two-way ANOVA P values are significant in regard to effects of diet and interaction. * P<0·05, HF different from MF and LF; †† P<0·01, ††† P<0·001, β-conglycinin different from control (casein).
Effects of β-conglycinin on faecal excretion of lipid
To reveal whether β-conglycinin lowered BW and liver TAG concentrations by up-regulating lipid excretion in faeces, faeces were collected and analysed. Faecal excretion of TAG was low corresponding to dietary fat levels (Table 4). However, β-conglycinin supplementation did not affect the faecal excretion of the TAG. The faecal TC concentration in the HF β-conglycinin dietary group was significantly low compared with that in the HF casein group indicating that β-conglycinin suppressed faecal excretion of TC. Taken together, β-conglycinin did not lower BW, the weight of WAT or liver TAG concentration due to the promotion of TAG excretion.
Table 4 Faeces TAG and total cholesterol (TC) concentrations(Mean values with their standard errors and two-way ANOVA P values, n 6)
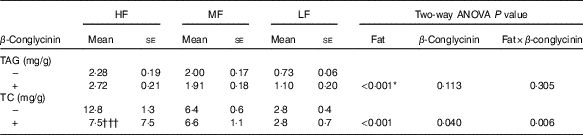
HF, high fat; LF, low fat; MF, medium fat.
* P<0·05, HF different from MF and LF. †††P<0·001, β-conglycinin different from control (casein).
Effects of β-conglycinin on energy production and physical activity levels in the high-fat dietary group
β-Conglycinin-supplemented mice in the HF dietary groups resulted in significantly lower BW compared with casein-fed mice, possibly by an alteration in energy production or substrate utilisation caused by β-conglycinin. Several factors were measured to examine the effects of β-conglycinin on the energy production and substrate utilisation of mice in the HF diet group (Table 5). There were no significant difference in VO2, RQ ratio, energy production, glucose and lipid production and daily activity levels during either the dark (feeding period) or light (sleeping period) cycle between mice fed the HF casein diet or the HF β-conglycinin diet.
Table 5 Effect of β-conglycinin on carbon dioxide production (VO2), RQ ratio and spontaneous motor activity of mice in high-fat (HF) dietary groups(Mean values with their standard errors, n 6)
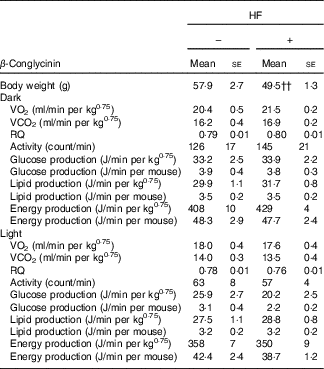
HF, high fat.
†† P<0·01, β-conglycinin different from control (casein).
Discussion
In this study, we showed that β-conglycinin ameliorates obesity and fatty liver of DIO mice and clarified the mechanism underlying these beneficial effects. We found that the expression of Pparγ2 in the liver and those of Pparγ1 and Pparγ2 in the subcutaneous WAT from β-conglycinin-fed mice were significantly suppressed compared with casein-fed mice in the HF dietary groups. Moreover, in the subcutaneous WAT, β-conglycinin also significantly suppressed the expressions of inflammation-related genes in the HF dietary group. The expression of Srebp-1c in the liver was significantly suppressed by β-conglycinin supplementation.
NAFLD is caused by an imbalance between TAG synthesis and removal in the liver( Reference Donnelly, Smith and Schwarzenberg 13 ). There is a marked increase in the fatty acid intake of the liver in mice fed an HF diet due to the elevated expression of Pparγ2 and the target genes( Reference Yamazaki, Shiraishi and Kishimoto 18 ). In the current study, we also observed these elevated expressions only in the HF dietary group and not in the MF and LF dietary groups. The actions of PPARγ are mediated by two protein isoforms, PPARγ1 and PPARγ2, and both are produced from a single gene by alternative splicing and differ only by an additional twenty-eight amino acids in the N terminus of PPARγ2( Reference Fajas, Auboeuf and Raspe 34 ). Pparγ1 levels were not altered in the livers of the DIO mice( Reference Yamazaki, Shiraishi and Kishimoto 18 ). We showed here that the hepatic expression levels of PPARγ2 and Cd36 were suppressed in the HF group by β-conglycinin to the same level as that in all of the other groups. The protein levels of PPARγ2 were also low in the β-conglycinin-supplemented group. Thus, the amelioration of the HF diet-induced NAFLD by β-conglycinin supplementation was caused by the decreased levels in protein and mRNA of PPARγ2. SREBP-1c is required in the activation of hepatic de novo lipogenesis( Reference Foretz, Pacot and Dugail 14 , Reference Horton, Bashmakov and Shimomura 15 ). We showed here that β-conglycinin significantly decreased the mRNA levels of SREBP-1c, FAS, SCD1 and ACC1. Insulin plays a critical role in lipogenesis by activating the expression and activity of SREBP-1c( Reference Foretz, Pacot and Dugail 14 ), and the concentration of serum insulin was low in the β-conglycinin-supplemented mice. Therefore, SREBP-1c mRNA was decreased because of the decreased serum insulin in the β-conglycinin-fed mice. Decreased PPARγ2 protein in the liver by knockdown of PPARγ2 mRNA reduced the expression of its target genes and de novo lipogenesis-related genes despite no alterations of SREBP-1c mRNA( Reference Yamazaki, Shiraishi and Kishimoto 18 ). It remains unclear whether PPARγ2 regulates the transcription of de novo lipogenesis-related genes directly or indirectly, but β-conglycinin seems to decrease the mRNA expressions of these genes at least in part through the decrease of Pparγ2 expression. Insulin also induces the expression of leptin in adipocytes( Reference Kim, Sarraf and Wright 35 ). Therefore, the decreased serum concentration of insulin caused by β-conglycinin leads to a decrease in the expression and secretion of leptin in WAT.
The expression of Pparα, a transcription factor responsible for fatty acid oxidation( Reference Lee, Pineau and Drago 36 ), was not affected by β-conglycinin supplementation. The increase of fatty acid oxidation might not be necessary for β-conglycinin to decrease the hepatic TAG concentration.
We reported previously that Pparγ2, Srebp-1c and their target genes were down-regulated when β-conglycinin prevented HF diet-induced fatty liver( Reference Yamazaki, Kishimoto and Miura 29 ). In the present study, we found that β-conglycinin improved the fatty liver of DIO mice due to the down-regulation of Pparγ2, Srebp-1c and their target genes. Thus, β-conglycinin might have both preventive and ameliorative effects through down-regulation of these genes.
PPARγ also has a critical role in the regulation of adipocyte differentiation and induces adipocyte hypertrophy by HF diet, and some functions such as lipogenesis appear to be governed exclusively by PPARγ ( Reference Tontonoz, Hu and Spiegelman 37 , Reference Kubota, Terauchi and Miki 38 ). We found that the mRNA expressions of PPARγ1 in the HF group and PPARγ2 in the HF and MF groups in subcutaneous WAT were suppressed by β-conglycinin to the same level as all the other groups. Moreover, β-conglycinin supplementation resulted in the lower mRNA expressions of aP2. However, we could not observe these changes in epididymal WAT. Thus, the decreased weight in subcutaneous WAT was coincident with the decreased expressions of Pparγ1/2.
Obesity is associated with chronic low-grade inflammation. Gene expressions of macrophage markers such as CD68 and F4/80 have been shown to be dramatically increased with obesity induced by HF diet feeding, especially in visceral WAT( Reference Weisberg, McCann and Desai 39 , Reference Ibrahim 40 ), but they were significantly decreased by β-conglycinin not only in epididymal WAT but also in subcutaneous WAT in the HF dietary group in our study. MCP-1 increases in the mature adipocyte fraction of obese mice and promotes monocyte infiltration into the WAT; these monocytes then differentiate into adipose tissue macrophages( Reference Osborn and Olefsky 41 ), of which there are two polarised states: M1 (proinflammatory macrophages) and M2 (anti-inflammatory macrophages)( Reference Martinez, Gordon and Locati 42 ). MCP-1 is one of the M1 macrophage markers( Reference Ohashi, Parker and Ouchi 43 ). Thus, β-conglycinin improved obesity partly due to the amelioration of inflammation in WAT.
WAT is an important endocrine organ that secretes a large number of adipocytokines, such as adiponectin and leptin, which are involved in a variety of physiological and pathological processes( Reference Hu, Liang and Spiegelman 44 , Reference Rousseau, Becker and Ongemba 45 ). Leptin is induced in WAT under an HF diet and secreted by subcutaneous WAT and visceral WAT, but it is secreted more from subcutaneous WAT than visceral WAT( Reference Rousseau, Becker and Ongemba 45 , Reference Van Harmelen, Reynisdottir and Eriksson 46 ). The results of our study agree with these previous studies in that the mRNA level in the subcutaneous WAT and serum concentrations of leptin in β-conglycinin-fed mice were drastically decreased. Adiponectin plays a role in adipogenesis and inhibits inflammation in adipose tissue( Reference Lovren, Pan and Quan 47 ). The plasma concentration of adiponectin is inversely related to BW( Reference Nakamura, Sekikawa and Kadowaki 48 ). The serum concentration of adiponectin in this study was not increased in the β-conglycinin-fed mice, despite their weight loss. Moreover, the mRNA expression of adiponectin in subcutaneous WAT was unexpectedly decreased in the β-conglycinin-supplemented HF diet-fed mice rather than in the β-conglycinin-unsupplemented HF diet-fed mice. Because visceral WAT is a more active producer of adiponectin than subcutaneous WAT( Reference Nakamura, Sekikawa and Kadowaki 48 ), the influence of β-conglycinin on the decreased expression of adiponectin in subcutaneous WAT appears to be small. Thus, the improvement effected by β-conglycinin did not include changes in adiponectin.
In humans, the estimated average intake of β-conglycinin is very low (<1 energy%). Mean intake of soyabeans and their related foods in Japanese was 58·6 g/d, and protein intake was 5·1 g( 49 ). Because 30 % of the soya protein was β-conglycinin, about 1·5 g of β-conglycinin was consumed per day on average( Reference Iwabuchi and Yamauchi 24 ). If we assume that average energy intake in humans is 8368 kJ/d (2000 kcal/d) and energy from protein is 17 kJ/g (4 kcal/g), then 1·5 g of β-conglycinin corresponds to 0·3 energy%. In humans, additional supplementation of 5 g of β-conglycinin was reported to effectively reduce intra-abdominal obesity( Reference Kohno, Hirotsuka and Kito 28 ). This amount corresponds to 1 energy%, which is lower than the dose used in this study, 20 energy%, required to elicit a lower WAT weight in ddY mice, suggesting that humans might be more sensitive to β-conglycinin than ddY mice. Although there is concern that β-conglycinin is an allergen, there is no mention of allergy in these reports( Reference Kohno, Hirotsuka and Kito 28 , Reference Wang, Qin and Sun 50 ).
Although the mechanism of how β-conglycinin affects lipid metabolism has not been identified, the peptide generated from β-conglycinin seems to have some role. It was revealed that small peptides released by the digestion of β-conglycinin activate LDL receptors and decrease the secretion of lipoprotein-containing apoB-100 in cultured HepG2 cells( Reference Lovati, Manzoni and Gianazza 51 , Reference Mochizuki, Maebuchi and Kohno 52 ). KNPQLR, EITPEKNPQLR and RKQEEDEDEEQQRE, three peptides from purified β-conglycinin hydrolysates, show FAS-inhibitory biological activity( Reference Martinez-Villaluenga, Rupasinghe and Schuler 53 ). Taken together, some peptides digested from β-conglycinin may affect lipid metabolism in the adipose tissue and liver directly or indirectly.
In this study, the mice fed a β-conglycinin-supplemented diet resulted in the lower weight of WAT, indicating that increased energy production might occur in these mice. However, we did not detect any changes in energy production or physical activity levels. This result is similar to that in our previous study, and it might be due to the sensitivity of indirect calorimetry( Reference Yamazaki, Kishimoto and Miura 29 ).
When severely obese DIO mice whose average BW was over 60 g were fed with β-conglycinin, there was no beneficial effect on NAFLD or obesity (data not shown). It seems that soya protein β-conglycinin ameliorated obesity and NAFLD only in mildly obese DIO mice. That is, β-conglycinin might be effective in overweight rather than obese subjects. Moreover, it seems that these beneficial effects of β-conglycinin were exclusively observed in the HF dietary group because the lower fat levels in the MF and LF groups themselves are beneficial for NAFLD and obesity.
In conclusion, dietary supplementation with β-conglycinin ameliorated NAFLD in DIO mice. β-Conglycinin suppressed the hepatic expression of Pparγ2 in the HF dietary group and also that of Srebp-1c. β-Conglycinin also effectively improved obesity in DIO mice accompanied by decreases in the expression of Pparγ1 in the HF dietary group and Pparγ2 in the HF and MF dietary groups and in the levels of serum insulin and leptin. β-Conglycinin may be a promising dietary protein for the amelioration of NAFLD and obesity.
Acknowledgements
This work was supported in part by a JSPS Grant-in-Aid for Scientific Research (C) grant number 25350918.
The author contributions are as follows: T. Y. designed the research; D. L. conducted the research; and D. L., R. I. and T. Y. analysed the data. D. L. and T. Y. wrote the manuscript with contributions from R. I. All authors have read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518000739














