Child mortality represents a major public health threat in sub-Sahara Africa. It was reported that in 2013, sub-Sahara Africa contributed 50 % to the overall worldwide mortality of children aged 0–59 months. In the same year, more than 8000 children died every day, amounting to approximately 3 million deaths annually(Reference Liu, Oza and Hogan1). Nutritional status is a major determinant of child mortality in low- and middle-income countries(Reference Ricci, Asare and Carboo2). Underweight, stunting and wasting, (defined as weight-for-age, height-for-age and height-for-weight below −2 z-scores of the median WHO growth standards), account for 14·4, 12·6 and 14·7 %, respectively, of deaths in the first 5 years in low- and middle-income countries(Reference Black, Victora and Walker3). Ghana is not an exception in sub-Sahara Africa. During the period 2009–2014, an overall mortality rate of 60 deaths per 1000 live births was registered in Ghana in children aged 0–59 months. Thus, one in every seventeen children does not reach his or her fifth birthday(4). Fortunately, undernutrition is currently decreasing in Ghana. According to the Ghana Demographic and Health Survey conducted in 2014, underweight, stunting and wasting decreased to 11, 19 and 5 %, compared with 18, 35 and 8 % in 2003(4). Regardless of this, undernutrition in Ghana remains a public health problem(5).
Severe acute malnutrition (SAM) is characterised by profound changes in body composition and physiology. In addition to the typical features of malnutrition, children often present with an array of complications and infections. Respiratory, urinary and gastrointestinal infections are common in children requiring admission for SAM(Reference Amadi, Kelly and Mwiya6–Reference Ricci, Carboo and Asare9). Infectious diseases, such as HIV, malaria and tuberculosis, are very common in Africa and worsen the already compromised clinical condition(Reference Gupta, Gupta and Atreja10–Reference Van Lettow, Fawzi and Semba14). Shock, anaemia and seizures are frequent complications in SAM and are associated with an increased risk of death(Reference Manary and Sandige15). There is, however, a scarcity of evidence regarding the impact of specific clustering of these risk factors in children with SAM.
An improved understanding of the complex interaction between these complications and infections as well as the relative importance of presenting clinical signs will assist in early identification and management of children at risk of dying. The primary aim of the study was therefore to determine risk factors for death and if specific clusters of risk factors are associated with increased mortality. Multivariate clustering techniques were used to disentangle the complex relationship between medical conditions (e.g. pneumonia and hypothermia) and clinical signs (e.g. pallor and eye signs suggestive of vitamin A deficiency) of children admitted for SAM and to define profiles or clusters of associations. Finally, the baseline profiles of medical conditions and clinical signs related to child mortality risk, time to mortality and time to discharge were also determined.
Methods
Sample and study design
The present study is a part of the Severe Acute Malnutrition in African Children (SAMAC) project, an ongoing programme of studies coordinated by the Centre of Excellence for Nutrition at the North-West University in South Africa. Briefly, data from selected SAM centres in different sub-Sahara African countries are collected by trained operators from existing medical records(Reference Carboo, Lombard and Conradie16). Data from eligible SAM medical records are extracted and recorded on a dedicated electronic database for further use. The data extraction is done with a formal data extraction tool, adapted from WHO admission criteria and treatment guidelines for children with SAM(17). For this analysis, a complete set of demographics, medical conditions, anthropometric measurements and vital signs at the admission as defined, measured and diagnosed by the clinician were collected. Data on mortality and discharge were also collected.
Children were included if they were born at term, were between the age of 0 and 59 months and diagnosed with SAM according to current WHO guidelines(18). Children with metabolic, neurodevelopmental or growth disorders unrelated to SAM or those who were re-admitted for SAM were excluded. The present work is based on a sample of 601 children with a first diagnosis of SAM, admitted and treated between June 2013 and June 2018, in three referral hospitals located in the Ashanti, Greater Accra and Northern regions of Ghana. The hospitals have malnutrition wards and accept SAM referrals from neighbouring and distanced hospitals.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving data extraction from medical records were approved by the Health Research Ethics Committee (NWU-00063-17-S1) at North-West University (South Africa) and Ghana Health Service Ethics Review Committee (GHS-ERC 09/06/17). Individual hospitals provided written permission.
Statistical analysis
Participant characteristics were described using medians and interquartile ranges if continuous; counts and percentages were used elsewhere. The z-scores of the median WHO growth standards for length/height-for-age, weight-for-age, weight-for-length/height, BMI and mid-upper arm circumference were performed, taking into account age and sex of the children, using the WHO SAS macro programme %igrowup_standard(19).
Two analyses were conducted to derive patterns of associations between infections (urinary tract, respiratory, and gastrointestinal infections, and malaria), infectious diseases (tuberculosis and HIV) and clinical signs (diarrhoea, oedema, convulsions, shock, vomiting, pallor, irregular heartbeat and weak pulse). Firstly, a correspondence analysis of the design matrix, a matrix having the subjects’ features as column, the subject as row and the yes/no coded as a 1 or 0, was conducted to derive a two-way map of the associations between those features. Afterwards, a hierarchical clustering analysis was conducted on the same matrix of associations among characteristics of children with SAM. Time to event analysis was undertaken to estimate mortality risks.
To this aim, features belonging to a single cluster were related to mortality risk using an accelerated failure model, based on the Weibull distribution. The accelerated failure model was chosen over the most common Cox proportional hazard model because the assumption of risk proportionality should not be assumed when considering children with SAM during emergency care. As a confirmation, we observed that mortality risk was not constant during the hospitalisation time and was particularly high in the first days after the admission resulting in a monotone decrease function of time. This is likely due to the early emergency care provided to children diagnosed with SAM. Briefly, in most cases, children with SAM are admitted to the hospital when their conditions are already critical and therefore need to be stabilised early. If stabilisation is successful during the first few days after the admission, then mortality generally decreases.
All survival models were adjusted for age, sex and treatment hospital. Finally, time to discharge and time to mortality were estimated using a singular random effect model adjusted for age and sex. This model had the logarithm of time to event as an outcome, hospital as a random factor and the clinical condition as a fixed effect. Least squares marginal means derived from the models were compared considering unequal sample sizes by group using the Tukey–Kramer adjustment(Reference Kramer20). Least squares marginal means was reported as retro-transformed exponentials resulting in geometric means. Sensitivity analyses were conducted including z-scores of the median WHO growth standards for length/height-for-age, weight-for-age, weight-for-length/height along with an indicator variable coding for missing values.
All statistical analyses were conducted using SAS software version 9.4. The multivariate clustering of the features of children with SAM was performed using the PROC CORRESP and the PROC VARCLUS. The LIFEREG and the GLM procedures were used to perform survival analysis and to estimate the time to outcome (mortality or discharge), respectively. All statistical tests were two-tailed, and the type-I error rate was set to 5 % (α = 0·05).
Results
The median age of the sample was 13 (interquartile range 9–22) months. Sixty-seven children were below the age of 6 months (11·1 %), 399 children were between 6 and 23 months (66·4 %) and 135 children were older than 24 months (22·5 %). The sample was well balanced with respect to sex being composed of 310 boys and 291 girls. The median length of stay was 11 d among survivors (interquartile range 8–17 d). Among the 601 children admitted and treated for SAM at the three hospitals, ninety-nine (16·5 %) died. The median time to death after admission was 5 (interquartile range 2–9) d.
Less than half of the children had oedematous malnutrition (n 239 (39·8 %)). The most common presenting clinical signs were diarrhoea and vomiting (n 303 (50·4 %) and n 292 (48·6 %), respectively). Sample characteristics at admission are reported in Table 1. The correspondence and hierarchical cluster analysis of medical conditions and clinical signs resulted in three comparable clusters of medical conditions and clinical signs (Fig. 1).
Table 1. Admission characteristics of children with severe acute malnutrition and distribution of clinical conditions by clusters of association for the total sample, children who survived and children who died
(Median values and minimum, maximum values; median values and interquartile ranges (IQR); numbers and percentages)
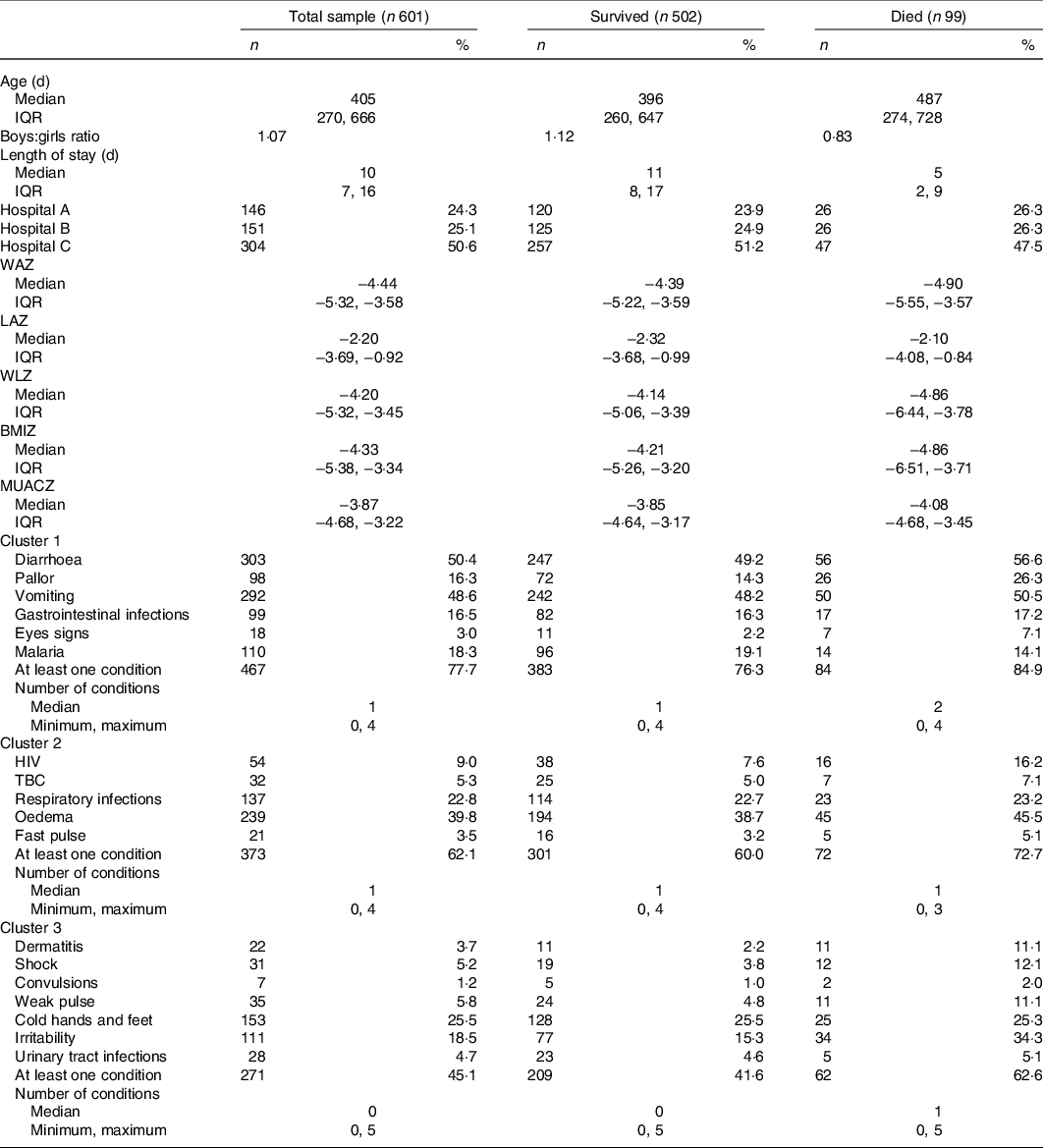
WAZ, weight for age z-score; LAZ, length for age z-score; WLZ, weight for length z-score; BMIZ, BMI for age z-score; MUACZ, middle up circumference for age z-score; TBC, tuberculosis.
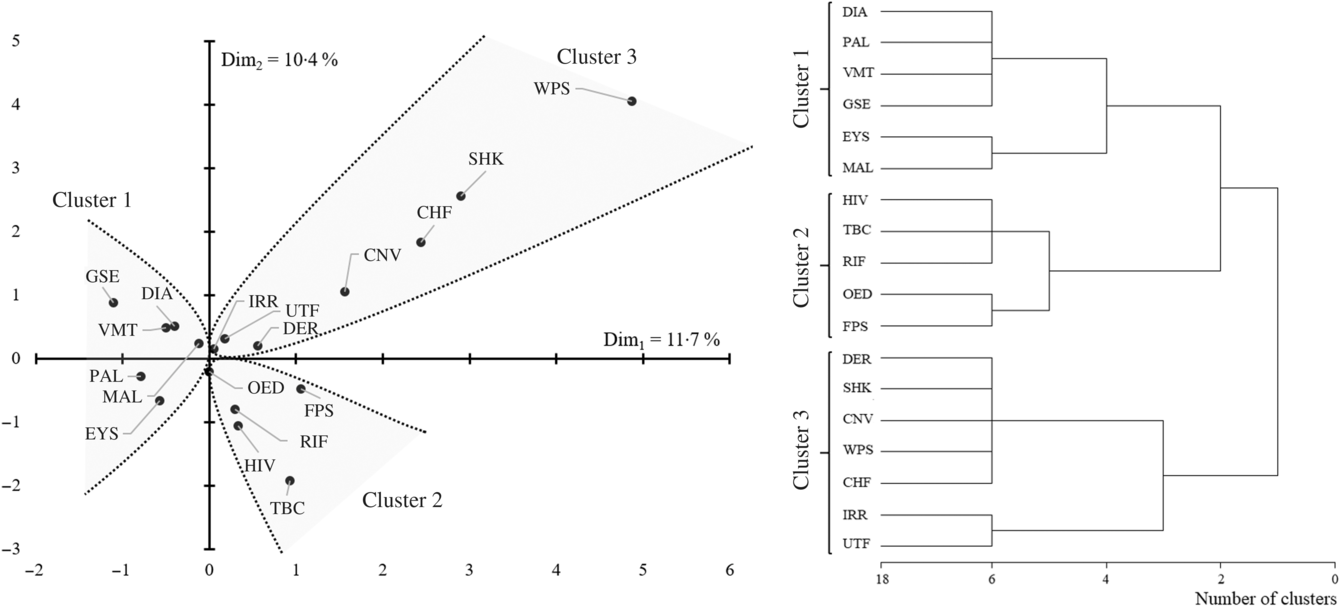
Fig. 1. Association map and hierarchical clustering of the 601 children with severe acute malnutrition clinical features. DIA, diarrhoea; PAL, pallor; VMT, vomiting; GSE, gastrointestinal infections; EYS, eye signs; MAL, malaria; TBC, tuberculosis; RIF, respiratory infection; OED, oedema; FPS, fast pulse; DER, dermatitis; SHK, shock; CNV, convulsions; WPS, weak pulse; CHF, cold hands and feet; IRR, irritability; UTF, urinary tract infection.
The first cluster of association was defined by eye signs due to vitamin A deficiency, pallor due to Fe deficiency anaemia and poor circulation, diarrhoea and vomiting with gastrointestinal infections and malaria. Among the features belonging to this cluster, we estimated that eye signs and pallor resulted in increased mortality risk of two to five times (hazard ratio (HR) 5·35, 95 % CI 1·63, 17·6 and HR 2·39, 95 % CI 1·17, 4·87, respectively). We estimated that an accumulation of any one of these features would result in approximately 30 % increased mortality risk (HR 1·32, 95 % CI 1·01, 1·73, Fig. 2). We also observed that, when present, diarrhoea and pallor may increase the length of stay by approximately 2 d among survivors (Table 2).
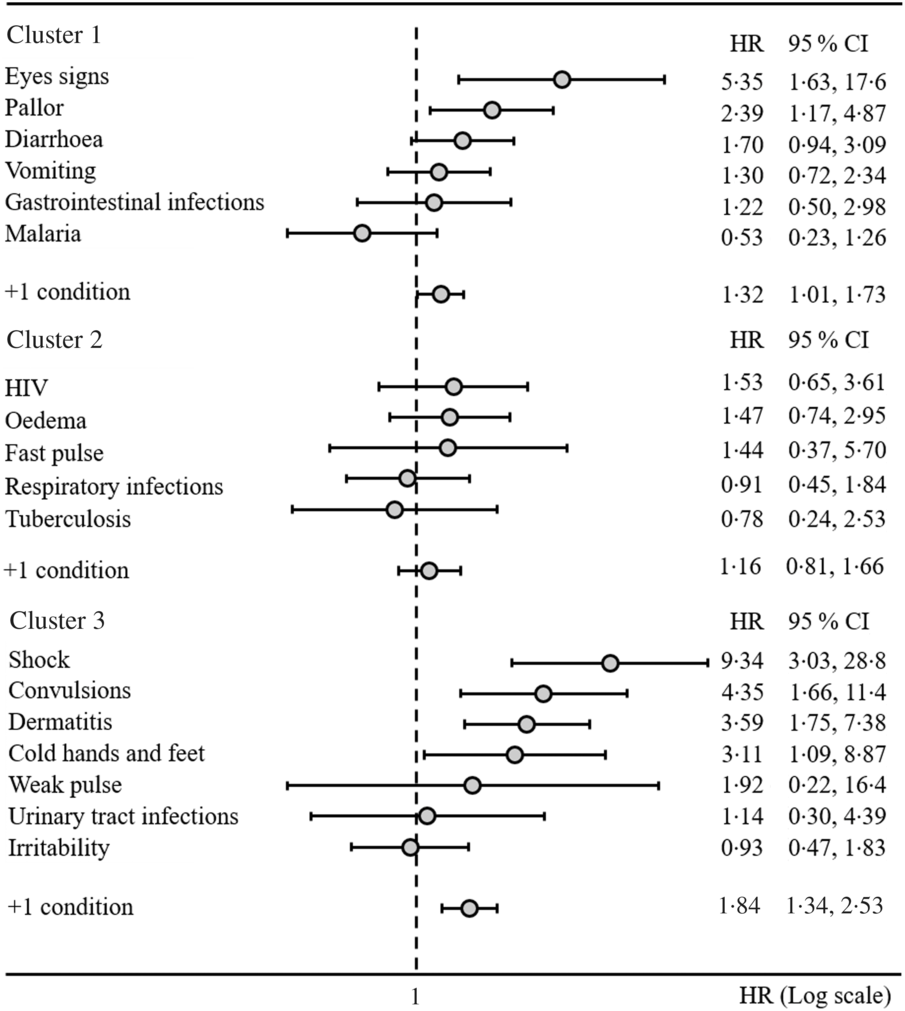
Fig. 2. Hazard ratios (HR) of mortality by cluster of associations among clinical features of the 601 children with severe acute malnutrition.
Table 2. Time to mortality and discharge for single clinical conditions and cluster of association in 601 Ghanaian children admitted and treated for severe acute malnutrition
(Median values and interquartile ranges (IQR))
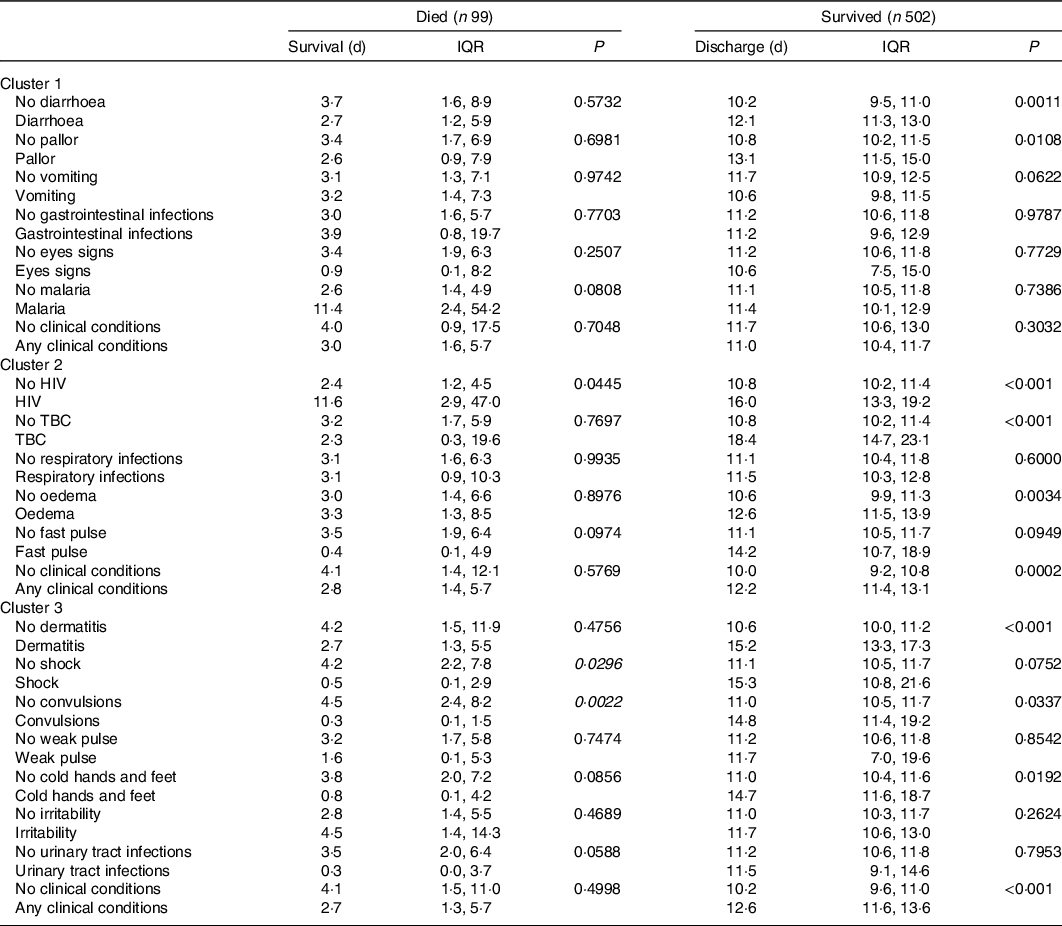
TBC, tuberculosis.
The second cluster of association was defined by HIV, oedema, fast pulse, respiratory infections and tuberculosis. None of the features belonging to this second cluster was significantly associated with increased mortality risk (Fig. 1). Nevertheless, we observed that HIV, tuberculosis and oedema are significantly associated with an increased length of stay among survivors. According to our estimates, HIV may increase the length of stay by approximately 5 d, whereas tuberculosis delays time to discharge by approximately 1 week. When present, oedema resulted in an increased length of stay of approximately 2 d (Table 2).
The third cluster of association was defined by shock (as defined by the admitting physician), convulsions, dermatitis, cold hands and feet, weak pulse, urinary tract infections and irritability. According to our analyses shock resulted in nine-time increased mortality risk (HR 9·34, 95 % CI 3·03, 28·8). Convulsions, dermatitis and cold hands and feet resulted in a three to four-time increased mortality risk (HR 4·35, 95 % CI 1·66, 11·4, HR 3·59, 95 % CI 1·75, 7·38 and HR 3·11, 95 % CI 1·09, 8·87, respectively). We estimated that an accumulation of any one of the conditions in the third cluster would result in 84 % increased mortality risk (HR 1·84, 95 % CI 1·34, 2·53, Fig. 2). Notably, when shock and convulsions were present, the time to mortality was reduced to approximately 4 d among children who died. Finally, dermatitis, convulsions and cold hands and feet delayed time to discharge among survivors in the range of 3–4 d (Table 2). Results were confirmed when sensitivity analyses considering z-scores and related indicator variables for missing values were included in the models.
Discussion
In the present study, we reported that admission medical diagnoses such as tuberculosis and clinical signs of children diagnosed, admitted and treated for SAM associate in patterns. These patterns were related to clinical outcomes influencing the length of stay among survivors, time to mortality and mortality risk despite the availability of internationally recommended SAM management protocols(Reference Ashworth, Khanum and Jackson21,22) . The first cluster contained children with signs of gastrointestinal infection, malaria and pallor. Hierarchical analysis further identified two sub-clusters, the first characterised by the diagnosis of gastrointestinal infection and its associated signs (diarrhoea and vomiting) together with pallor, and the second cluster grouping eye signs and malaria. Despite attempts to control malaria infection, it remains endemic in Ghana and a leading cause for hospital admission and death(Reference Darko, Tetteh and Ayanore23). The first sub-cluster is common in Africa due to poor hygiene conditions(Reference Fletcher, Stark and Ellis24,Reference Armah, Pager and Asmah25) . Gastrointestinal infections, whether viral or bacterial, are often associated with diarrhoea and vomiting(Reference Armah, Pager and Asmah25–Reference Reither, Ignatius and Weitzel27). The present study failed to find an increased risk of death associated with the clinical conditions in the first cluster, probably due to missing information in the medical records of children in our sample. For instance, a large number of participants in the present study had missing information on a rapid diagnostic test for malaria (n 491 (81·7 %)). Thus, it is unclear to what extent it contributes to the mortality of children with SAM. This is consistent with a study conducted in Ghana, where a high rate of missing data was reported for malaria and hypoglycaemia(Reference Asare28). Furthermore, we observed an association between those diagnosed with malaria and eye signs. Although vitamin A status was not formally assessed, the most frequent cause of eye signs in children with SAM is vitamin A deficiency, of which the current prevalence in Ghana is estimated at 20·8 % in children aged 6–59 months(29). Notably, acute infection, such as that caused by the malaria parasite, has been reported to reduce serum retinol concentration in children resulting in the inflammatory response(Reference Rosales, Topping and Smith30). Low serum retinol concentrations occur more frequently during peak malaria seasons(Reference Semba and Bloem31,Reference Barffour, Schulze and Coles32) . We are, however, uncertain to what extent the inflammatory response induced by malaria in this sample of patients contributed to vitamin A deficiency and its associated eye signs. Nevertheless, this may provide a possible explanation for the observed association in the present study. We further investigated the joint association of eye signs with malaria. We then evaluated a supplementary model having an interaction term for eye signs and malaria. In this analysis, the significance for eye signs was maintained while malaria and the interaction term were not associated with an increased mortality risk, confirming the prominence of vitamin A deficiency over malaria. Assuming that the eye signs were mostly due to vitamin A deficiency, the increased mortality in this cluster is in keeping with previous studies(Reference Fatima, Tariq and Qamar33,Reference Richa, Alka and Ranu34) . Accordingly, eye signs and pallor are readily assessed at the bedside and are among WHO-recommended priority signs which identify children at high risk of death if not treated in time(22). Studies conducted in sub-Sharan Africa reported a two- to five-time increased risk of death for children with SAM presenting with pallor(Reference De Maayer and Saloojee35,Reference Jarso, Workicho and Alemseged36) .
We further reported the second cluster of features given by the association of infectious diseases, such as HIV, respiratory infections and tuberculosis with clinical signs such as oedema and fast pulse. According to our hierarchical clustering, two sub-clusters can be defined within this cluster: a sub-cluster of diseases defined by the association between HIV, tuberculosis and respiratory infections and the second sub-cluster of clinical signs defined by the association between oedema and fast pulse. The association between HIV, tuberculosis and respiratory infections is common among children diagnosed with SAM in sub-Sahara Africa and has been widely described in scientific literature(Reference Bunn, Thindwa and Kerac37–Reference Jones and Berkley39). An association of these infections with oedema and fast pulse has also been reported in the literature(Reference Girma, Kæstel and Mølgaard40,Reference Coulthard41) . Features associated with this cluster were not related to an increased mortality risk, but we reported that they may increase the time to discharge in a relevant way, and this is consistent with previous studies findings(Reference Desyibelew, Fekadu and Woldie42,Reference Wagnew, Dejenu and Eshetie43) . We observed that mortality among children with SAM infected by HIV was approximately two times higher with respect to non-HIV infected children (sixteen deaths over ninety-nine (16·1 %) v. thirty-eight over 502 (7·6 %)). This supports previous studies conducted in sub-Sahara Africa(Reference Jarso, Workicho and Alemseged36–Reference Nabukeera-Barungi, Grenov and Lanyero44).
We analysed mortality risk of HIV-infected children using logistic regression adjusted for age, sex and hospital and confirmed a significant increase in mortality among HIV-infected children compared with non-HIV infected children (OR 2·22, 95 % CI 1·15, 4·32). Furthermore, HIV-infected children with SAM are more likely to die late than HIV-uninfected children with SAM, mostly after the first 48 h. These late deaths in HIV-infected children with SAM may reflect failure to control infection or metabolic derangements in these vulnerable children after admission or an increased risk of nosocomial infections(Reference Trenor45).
Finally, we observed a third cluster formed by the association of shock, convulsions, dermatitis, cold hands and feet and a weak pulse (reflecting poor peripheral perfusion and cardiac output), irritability and urinary tract infections. The mortality of children in this cluster was three to nine times greater than that of other children. This is congruent with other studies which reported that medical conditions such as shock resulted in a five- to eight-time increased mortality risk in children with SAM(Reference De Maayer and Saloojee35,Reference Wagnew, Tesgera and Mekonnen46) . These children also had a shorter survival time and survivors had prolonged hospitalisation. Shock and convulsions represented the most important indexes of increased mortality risk and reduced survival time. Notably, shock, cold hands and feet, weak and fast pulse, and convulsion are among the WHO-recommended emergency signs predicting early death(22). Features belonging to this cluster are commonly observed in children diagnosed with SAM and have previously been recognised as determinants of mortality in children with SAM, especially in association with infections and dehydration(Reference Jarso, Workicho and Alemseged36,Reference Jones and Berkley39,Reference Chisti, Salam and Bardhan47–Reference Tariq, Naik and Rafiq50) . In the present work, we confirmed that shock, convulsions, dermatitis and cold hands and feet are serious underlying conditions indicating the presence of septic shock. This has an extremely high mortality risk in children with SAM(Reference Wagnew, Tesgera and Mekonnen46,Reference Tariq, Naik and Rafiq50) .
The present study has many strong points. Firstly, it is based on a robust methodology considering a much more suitable survival model, without any assumption regarding hazard proportionality. We believe that, even if the hazard proportionality was ascertained, it is very unlikely in such an emergency setting. Notably, evaluating hazard proportionality with small sample size (n < 500) might result in high false-negative result rates, especially in the case when hazards are monotonous with respect to observational time. In this case, the accelerated failure models are a valid alternative(Reference Wei51). The present study adds certain relevant information regarding the determinants of mortality in Ghanaian children with SAM. Moreover, we not only evaluated single factors in relation to mortality, but different clinical features were evaluated as a whole, showing also how they associate with each other. In this sense, the present work is unique. Nevertheless, very few studies have been conducted to evaluate mortality in Ghanaian children with SAM. Finally, the present work did not only focus on mortality risks but also on time to mortality. We provided some useful information regarding the length of stay for those children with SAM who survived. We believe that these results may guide clinicians’ decision regarding priorities in managing children admitted to hospital for SAM.
The present work also has some limitations. Mainly, we acknowledge that the emergency setting in which the study was conducted may have led to some bias. In particular, we observed no increased mortality risk for certain infections, such as tuberculosis and malaria. In the present work, we observed a high number of missing information in the medical records, possibly because of the scarce workforce dedicated to completing admission forms. It is not possible for us to exclude that this factor, along with the emergency setting in which the data were recorded in the folder, may have led to a likely underreporting and misreporting of children clinical features.
Conclusions
Medical conditions and clinical signs in children with SAM associate in patterns. These patterns are strongly related to clinical outcomes and should be carefully considered in clinical practice. In the present study, we showed that clinical signs, more than single diseases, increased mortality outcomes. More specifically, shock, eye signs, convulsions, dermatitis, hypothermia and pallor were associated with higher mortality risk and reduced survival time among the 601 Ghanaian children evaluated.
Acknowledgements
The authors thank all the data extractors who made this work possible.
The Nutricia Research Foundation provided financial support for the data collection of the MSc student involved in the present study. The Nutricia Research Foundation had no role in the design, analyses and interpretation of analyses or writing of the article.
H. A. and C. R. conceived the work, performed statistical analysis and performed the first version of the draft. H. A. and J. C. performed data collection and designed the electronic form for data collection. E. N., R. C. D., C. C. and M. L. defined the protocol and the questionnaire for data collection. Each author actively contributed to the work.
None of the authors had any conflict of interest to declare.









