Article contents
Intestinal absorption of D-fructose isomers, D-allulose, D-sorbose and D-tagatose, via glucose transporter type 5 (GLUT5) but not sodium-dependent glucose cotransporter 1 (SGLT1) in rats
Published online by Cambridge University Press: 03 May 2023
Abstract
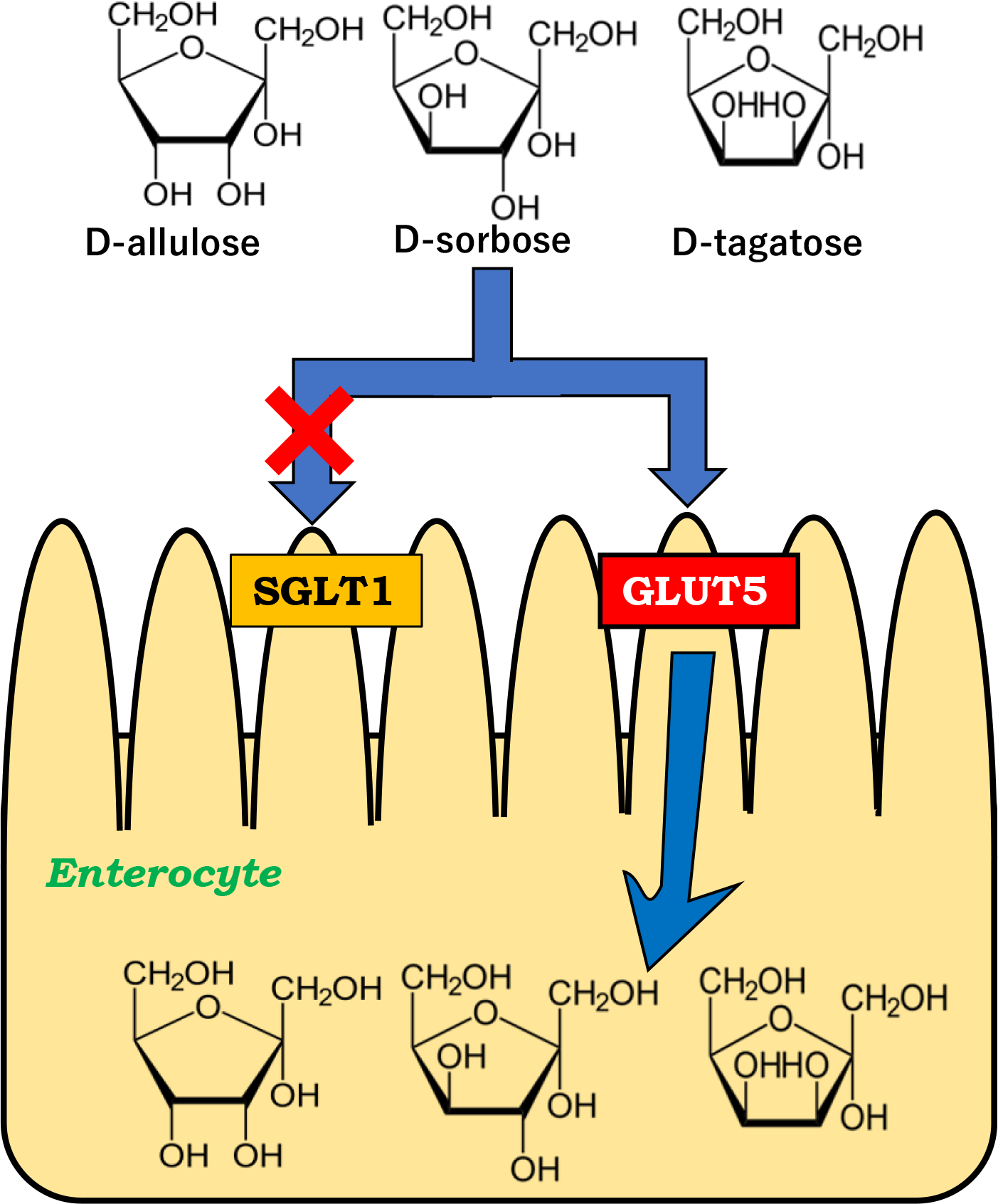
D-allulose, D-sorbose and D-tagatose are D-fructose isomers that are called rare sugars. These rare sugars have been studied intensively in terms of biological production and food application as well as physiological effects. There are limited papers with regard to the transporters mediating the intestinal absorption of these rare sugars. We examined whether these rare sugars are absorbed via sodium-dependent glucose cotransporter 1 (SGLT1) as well as via GLUT type 5 (GLUT5) using rats. High-fructose diet fed rats, which express more intestinal GLUT5, exhibited significantly higher peripheral concentrations, Cmax and AUC0–180 min when D-allulose, D-sorbose and D-tagatose were orally administrated. KGA-2727, a selective SGLT1 inhibitor, did not affect the peripheral and portal vein concentrations and pharmacokinetic parameters of these rare sugars. The results suggest that D-allulose, D-sorbose and D-tagatose are likely transported via GLUT5 but not SGLT1 in rat small intestine.
- Type
- Research Article
- Information
- Copyright
- © The Author(s), 2023. Published by Cambridge University Press on behalf of The Nutrition Society
References
- 2
- Cited by





