A recent study showed that long-term haemodialysis (HD) patients consumed fish far below current American Heart Association recommendationsReference Friedman, Moe, Perkins, Li and Watkins1, which was associated with a decreased plasma and erythrocyte membrane content in long-chain n-3 PUFA. In addition, a multivariate analysis carried out in 216 dialysis patients showed that patients who reported fish intake had an approximately 50 % lower risk of death at 1 yearReference Kutner, Clow, Zhang and Aviles2. Cardiac disease is the major cause of death in dialysis patients; approximately 20 % of cardiac deaths are attributed to acute myocardial infarctionReference Sarnak, Levey and Schoolwerth3. Chronic renal failure is characterized by a generalized vasculopathyReference Luke4. Increased sympathetic toneReference Zoccali, Mallamaci and Parlongo5–Reference Kotanko8 and insulin resistanceReference Rigalleau and Gin9 are, among a great variety, two risk factors that may contribute to the accelerated cardiovascular morbidity in patients undergoing HD for end-stage renal disease.
Long-chain n-3 PUFA, given as an oral fish oil supplementation, prevent the increase in sympathetic activity elicited by mental stress in healthy subjectsReference Delarue, Matzinger, Binnert, Schneiter, Chiolero and Tappy10. Fish oil also reduces by 40 % the insulinaemic response to oral glucose in healthy subjects without altering plasma glucose utilization, strongly suggesting an insulin-sensitizing effectReference Delarue, Couet, Cohen, Brechot, Antoine and Lamisse11. Moreover, fish oil supplementation partially prevents dexamethasone-induced insulin resistance in healthy subjectsReference Delarue, Chang-Hong, Cohen, Corporeau and Simon12. Taken together, these data suggest that long-chain n-3 PUFA could be useful in HD patients with increased sympathetic tone and insulin resistance.
The study of metabolic response to an oral glucose load before and after fish oil supplementation allows quantification of effects of long-chain n-3 PUFA on both sympathetic activity and whole-body glucose metabolism. Indeed, thermogenic response, i.e. increase in energy expenditure (EE), following glucose absorption includes a facultative component due to sympathoadrenal activationReference Acheson, Jequier and Wahren13, Reference Acheson, Ravussin, Wahren and Jequier14. Moreover, insulin sensitivity can be assessed from concomitant determinations of plasma glucose and insulin concentrations following oral glucoseReference Matsuda and DeFronzo15 and from determination of plasma glucose utilization by using stable isotope labelling of plasma glucoseReference Delarue, Couet, Cohen, Brechot, Antoine and Lamisse11.
The present work aimed to study whether or not a 3-week dietary fish oil supplementation (EPA + DHA at 1·8 g/d) modulates sympathoadrenal activity and/or whole-body glucose metabolism during an oral glucose load in HD patients.
Subjects and methods
Subjects
Eight patients (six men, two women, 62 (sem 7) years, 68·5 (sem 3·7) kg, BMI 24·7 (sem 1·8) kg/m2) undergoing HD for at least 6 months for end-stage renal disease were included. Aetiologies of end-stage renal disease were: polycystic disease of kidney (two), nephroangiosclerosis (one), tuberculosis (one), chronic interstitial nephropathy (one), chronic glomerulonephritis (one), post-traumatic nephropathy on unique kidney (one) and one of unknown cause. Mean duration of HD at day of inclusion in the study was: 7·5 (sem 3) years (n 8). No patient had personal or familial history of diabetes and/or obesity. None was taking any drug that could alter glucose metabolism or sympathetic activity.
Each subject had given written consent. The protocol was submitted and accepted by Ethical Committee of Brest.
Protocol
Patients were studied twice before and after a 3-week dietary supplementation with 6 g/d of a fish oil containing 18 % EPA and 12 % DHA (Maxepa®; Pierre Fabre, Castres, France), i.e. an intake of 1·8 g/d of EPA + DHA. Fish oil was given as two caps three times per d. Its composition is reported in Table 1.
All tests were started at 07·00 hours after patients had experienced a 12 h overnight fast. The patients were weighted, voided if non-anuric, and were placed at rest in a bed and maintained in the supine position all over the experiment. An intravenous catheter was inserted into a superficial wrist vein for blood sampling. Another catheter was placed into a deep controlateral forearm vein for tracer infusion. A primed-constant infusion of d-[6,6-2H2]glucose was started 150 min before the oral load and maintained for 510 min. The rate of tracer infusion was 70 μg/kg per min. All patients absorbed over 5 min 1 g glucose/kg diluted into 300 ml water (time 0 min). Blood samples were sequentially obtained for determination of plasma glucose, insulin, metabolite concentrations and isotopic enrichment in 2H of plasma glucose in the basal state and following glucose absorption. Plasma catecholamines were determined at times 180, 210, 240, 300 and 360 min.
Indirect calorimetry was performed using a canopy (Deltatrac Metabolic Monitor II; Datex Omheda, Helsinki, Finland). The patients wore the canopy during 1 h prior to the oral load, during the 3 h following the load and during 30 min for each of the remaining 3 h. Urine was collected at the end of the experiment only in non-anuric patients.
Sampling procedures and analytical methods
Blood samples were immediately spun at 4°C. Plasma was aliquoted and frozen at − 80°C until time of assay. Urine samples were collected and frozen at − 20°C for further determination of urinary urea. Plasma samples (1 ml) were extracted with 12 ml CHCl3–MeOH (2:1, v/v) according to the method described by Folch et al. Reference Folch, Lees and Sloane Stanley16. The final lipid extract was stored at − 20°C after addition of 0·01 % butylated hydroxytoluene (antioxidant). Neutral lipids were separated from lipid extract on a silicagel microcolumn (Kieselgel 60; 70–230 mesh; Merck; 10 ml CHCl3–MeOH (98:2, v/v)) according to Marty et al. Reference Marty, Delaunay, Moal and Samain17. TAG and NEFA were isolated by HPLC from the neutral lipid fraction as previously described by Soudant et al. Reference Soudant, Chu and Marty18 on a diol column (OH-bound silica gel column, Lichrosorb Diol 5 μm, 250 × 4 mm i.d.; Merck) using a binary mobile phase (mixtures of hexane and isopropanol). TAG and NEFA fractions were analysed for fatty acid composition using GC, after transesterification with MeOH–BF3 as previously describedReference Marty, Delaunay, Moal and Samain17. Fatty acid composition of fish oil was also analysed by the same methodReference Marty, Delaunay, Moal and Samain17.
Plasma glucose concentrations were measured using a YSI 2300 STAT Plus Glucose & Lactate Analyzer (Ysi Life Sciences, Yellow Springs, USA). Plasma NEFA were determined by an enzymatic colorimetric method using a commercial kit (NEFA C; Wako Chemicals, Neuss, Germany). Plasma immunoreactive insulin concentrations were measured by RIA (INSIK5; CIS Bio International, Gif/Yvette, France). Plasma catecholamines were determined by HPLC.
The enrichments in 2H were determined on the pentacetate derivative of glucose as previously describedReference Delarue, Couet, Cohen, Brechot, Antoine and Lamisse11.
Calculations
Protein oxidation was estimated from urinary urea excretion corrected for variation of body urea poolReference Jequier, Acheson and Schutz19. In anuric patients, protein oxidation was estimated from variation of body urea pool as previously describedReference Delarue, Maingourd, Lamisse, Garrigue, Bagros and Couet20.
Net carbohydrate and lipid oxidations were calculated by using the equations of Livesey & EliaReference Livesey and Elia21. Non-oxidative glucose disposal, i.e. mainly glycogen storage, was calculated as the difference between the amount of glucose absorbed and carbohydrate oxidation cumulated over the 6 h following glucose absorption.
Total rate of plasma glucose appearance (RaT) and disappearance (RdT) following glucose absorption were calculated from the isotopic enrichment in 2H of plasma glucose, by using Steele's equationReference Steele, Wall, De Bodo and Altszuler22 as modified by De Bodo et al. Reference De Bodo, Steele, Altszuler, Dunn and Bishop23.
Statistical methods
All results are expressed as means and their standard errors. The comparison between the periods with and without fish oil was made by a two-factor ANOVA with repeated measures and a two-tailed paired Student's t test where appropriate. Differences were regarded as significant when P < 0·05.
Results
Fatty acid content of plasma TAG
EPA and DHA content of plasma NEFA and TAG increased significantly after fish oil supplementation (Fig. 1), demonstrating a good compliance of patients for fish oil intake.

Fig. 1 Content in EPA (20 : 5n-3) and DHA (22 : 6n-3) of plasma TAG (a) and NEFA (b) in haemodialysis patients before (□) and after (■) an oral 3-week fish oil supplementation. Values are means with their standard errors depicted by vertical bars. Mean values were significantly different from those before supplementation: *P < 0·05, **P < 0·01.
Thermogenic response to glucose and plasma catecholamines
Resting EE was not different before and after fish oil supplementation (0·0946 (sem 0·0029) v. 0·0995 (sem 0·0598) kJ/kg fat-free mass per min, respectively (0·0226 (sem 0·0007) v. 0·0238 (sem 0·0143) kcal/kg fat-free mass per min, respectively)). Before fish oil supplementation, EE increased after glucose oral absorption from time 0 to time 60 min (P < 0·05), decreased from time 60 min until time 240 min, then re-increased abruptly from time 240 to time 360 min (P < 0·05) (Fig. 2). Plasma catecholamines increased concomitantly to the re-increase in EE (Fig. 2). Five patients displayed symptoms evocative of sympathetic overactivity, i.e. tremulousness, palpitations, anxiety and sweating.

Fig. 2 Thermogenic response (a), and plasma epinephrine (b) and norepinephrine (c) concentrations following a 1 g/kg glucose load in haemodialysis patients before (○) and after (●) an oral 3-week fish oil supplementation. Values are means with their standard errors depicted by vertical bars. (a), Mean values were significantly different from those at time 240 min: *P < 0·05. (b, c) Mean values were significantly different from those at time 210 min: *P < 0·05. EE, energy expenditure.
After fish oil, contrarily to what was observed before fish oil, no re-increase in EE was observed from time 240 to time 360 min (Fig. 2). Contrarily to what was observed before fish oil, plasma epinephrine did not increase from 180 to 240 min after fish oil (Fig. 2). Plasma norepinephrine after fish oil remained similar to before fish oil supplementation (Fig. 2). Symptoms of sympathetic overactivity remained unchanged in the same five patients after fish oil supplementation.
Substrate oxidation
Basal carbohydrate oxidation was similar before and after fish oil (1·68 (sem 0·27) v. 1·66 (sem 0·19) mg/kg per min, respectively). Basal lipid oxidation was also similar before and after fish oil (0·99 (sem 0·10) v. 1·19 (sem 0·10) mg/kg per min, respectively). Cumulated carbohydrate and lipid oxidation and non-oxidative glucose disposal were similar before and after fish oil supplementation (Table 2).
Table 2 Cumulated substrate oxidation, non-oxidative glucose disposal (NOGD) and plasma glucose fluxes (Mean values with their standard errors)
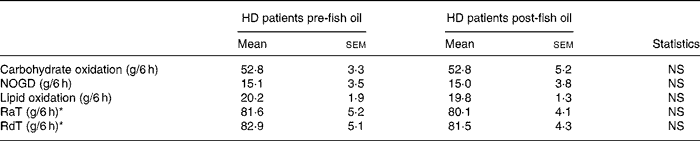
HD, haemodialysis; RaT, total rate of plasma glucose appearance; RdT, total rate of plasma glucose disappearance.
* To obtain in mol/6 h, divide by 180.
Metabolites and insulin
Basal concentrations of plasma glucose, NEFA and insulin are reported in Table 3. Kinetics of glycaemia, insulin and NEFA were similar before and after fish oil supplementation (Fig. 3).
Table 3 Basal concentrations of plasma metabolites and insulin (Mean values with their standard errors)

HD, haemodialysis patients.
* To obtain in g/l divide by 5·55.
† To obtain in mU/ml, divide by 6.

Fig. 3 Plasma glucose (a), insulin (b) and NEFA (c) concentrations in response to a 1 g/kg glucose load in haemodialysis patients before (●) and after (○) an oral 3-week fish oil supplementation. Values are means with their standard errors depicted by vertical bars.
Plasma glucose fluxes
Basal RaT was not different before and after fish oil supplementation (2·14 (sem) 0·13 v. 2·04 (sem 0·07) mg/kg per min, respectively). Cumulated RaT and RdT were not different before and after fish oil (Table 2).
Discussion
The main result of the present study is the marked attenuation by fish oil of the adrenergic overactivity induced by oral glucose in the HD patients. The kinetics of thermogenic response to oral glucose before fish oil supplementation was characterized by a re-increase in EE during the last 2 h of the study. This re-increase in EE coincided with both an increase in plasma catecholamines, mainly epinephrine, and symptoms (in five of the eight patients) evocative of sympathetic overactivity, i.e. tremulousness, palpitations, anxiety and sweating, looking like neurogenic symptoms of hypoglycaemiaReference Cryer24. Taken together, these present observations strongly suggest a sympathoadrenal activation in the patients in response to oral glucose. This sympathoadrenal activation was likely triggered by the abrupt decrease in glycaemia from time 120 to time 240 min. The fact that the hypoglycaemia threshold was far from being reached strongly reinforces the hypothesis of a sympathoadrenal overactivity in HD patients as previously shown by othersReference Zoccali, Mallamaci and Parlongo5–Reference Kotanko8. These neurogenic symptoms are very unusual during an oral glucose load; they are typically observed only in non-diabetic subjects with reactive hypoglycaemia.
Following fish oil, the re-increase in EE over the last 2 h of the experiment was completely blunted, in parallel to the blunting of the increase in plasma epinephrine concentrations. As the increase in plasma norepinephrine concentrations remained unaffected by fish oil, the re-increase in EE was very likely specifically linked to adrenergic activation in HD patients. It has been shown in healthy subjects that the facultative thermogenic response to glucose was due to adrenergic activationReference Muller, Acheson, Piolino, Jeanpretre, Burger and Jequier25. The blunting of adrenergic response by fish oil has been previously reported in healthy subjects during mental stressReference Delarue, Matzinger, Binnert, Schneiter, Chiolero and Tappy10. Whether fish oil blunted the re-increase in EE, it did not prevent the neurogenic symptoms of sympathetic activation. As neurogenic symptoms associated to hypoglycaemia are mainly related to sympathetic activation rather than adrenal activationReference DeRosa and Cryer26, on the one hand, and as circulating norepinephrine is derived largely (9–98 %) from sympathetic postganglionic neuronsReference Brown, Jenner, Allison and Dollery27, Reference Hoeldtke, Cilmi, Reichard, Boden and Owen28, on the other hand, it can be concluded that in the HD patients fish oil attenuated specifically adrenergic overactivity.
Fish oil altered neither glycaemic and insulin responses to oral glucose nor plasma glucose utilization, carbohydrate oxidation and non-oxidative glucose disposal. This contrasts with what has been reported in healthy subjects. Indeed, fish oil decreased by 40 % the insulinaemic response to oral glucoseReference Delarue, Couet, Cohen, Brechot, Antoine and Lamisse11 and partially prevented the excessive increase in insulinaemia induced by a 2 d dexamethasone treatmentReference Delarue, Chang-Hong, Cohen, Corporeau and Simon12. It can be hypothesized that fish oil was ineffective in decreasing insulinaemic response to oral glucose in the HD patients because of a pre-existing insulin resistance. Indeed, it has been repeatedly shown that fish oil supplementation was unable to reverse insulin resistance in patients with type 2 diabetes (reviews in Delarue et al. Reference Delarue, LeFoll, Corporeau and Lucas29 and Lombardo & ChiccoReference Lombardo and Chicco30). As HD patients generally display insulin resistanceReference Rigalleau and Gin9, the lack of effect of fish oil could be interpreted as its inability to reverse insulin resistance once installed. Data in rodents also sustain the inability of fish oil to reverse insulin resistance whereas it is able to prevent its installation (reviews in Delarue et al. Reference Delarue, LeFoll, Corporeau and Lucas29 and Lombardo & ChiccoReference Lombardo and Chicco30).
The lack of randomization of fish oil intake in the present study is unlikely to explain its effect on the post-glucose kinetics of EE and plasma epinephrine. A cross-over study would have been an ideal design, but EPA and DHA incorporation into membranes has been reported to be as long as 18 weeksReference Endres, Ghorbani and Kelley31 so that the two experiments would have to be performed at least 18 weeks apart. During such a long period other confounding factors could appear, especially in HD patients. Patients had very similar glycaemia, insulinaemia, substrate oxidation and plasma glucose fluxes in the basal state and after glucose without and with fish oil supplementation. In addition, re-increase in EE during the last part of the oral load appears to be specific to our HD patients inasmuch as it has never been reported before, especially in healthy subjects and in patients undergoing continuous ambulatory peritoneal dialysis (CAPD) that we previously studied using the same methodologyReference Delarue, Couet, Cohen, Brechot, Antoine and Lamisse11, Reference Delarue, Chang-Hong, Cohen, Corporeau and Simon12, Reference Delarue, Maingourd, Lamisse, Garrigue, Bagros and Couet20, Reference Delarue, Maingourd, Couet, Vidal, Bagros and Lamisse32. Thus, we are very confident that attenuation by fish oil of adrenergic overactivation was not due to the lack of randomization of its administration. It is to be noted that in contrast to haemodialysed patients from the present study and others, sympathetic activity has been reported to be impaired in patients undergoing CAPDReference Jassal, Allen, Douglas and Stout33. This could explain why we did not observe any neurogenic symptom previously in CAPD patients absorbing an oral glucose loadReference Delarue, Maingourd, Lamisse, Garrigue, Bagros and Couet20, Reference Delarue, Maingourd, Couet, Vidal, Bagros and Lamisse32. The mechanisms sustaining a decreased sympathetic activity on CAPD remain unclear. It could be possible that chronic peritoneal glucose administration (glucose being used as an osmotic agent) could have deleterious effects comparable to that observed in patients with diabetes.
Sympathetic overactivity has been repeatedly observed in patients with end-stage renal disease undergoing HD. An increased adrenergic response in everyday life induces at least chronic vasoconstriction leading to hypertension and to endothelial dysfunction leading itself to atherosclerosis. In addition, insulin resistance is a common metabolic disorder in patients with end-stage renal disease, leading to increased cardiovascular risk, predisposing to metabolic syndrome and type 2 diabetes and stimulating Na+ renal reabsorption favouring hypertension. Fish oil has many beneficial effects: reduction of cardiovascular risk, reduction of adrenergic overactivity induced by mental stress and prevention of insulin resistance. For all those reasons, fish oil supplementation could be very useful in HD patients. We did not observe any effect on insulin sensitivity and others did not observe any effect on heart rate variabilityReference Svensson, Schmidt, Jorgensen and Christensen34, suggesting that fish oil is not able to correct all metabolic alterations in HD patients, but the decreased sympathetic overactivity is of potential major interest to reduce vasoconstriction, risk of hypertension and endothelial dysfunction and in longer term to reduce cardiovascular risk. Of course, further studies are needed to confirm in HD patients these possible beneficial long-term effects.
In conclusion, a fish oil supplementation containing EPA + DHA at 1·8 g/d over 3 weeks attenuates adrenergic overactivity in response to oral glucose in HD patients without altering whole-body glucose metabolism and insulin sensitivity.
Acknowledgements
The technical help of Mrs Yvette Creff and Véronique Troadec was deeply appreciated. We thank Mr M. Desage from Centre de Recherche en Nutrition Humaine de Lyon for performing the analysis of isotopic enrichment in 2H of plasma glucose, Mrs M. P. Moineau (Service de Médecine Nucléaire, CHU de Brest) for performing the assays of plasma insulin and Mr J. M. Cottet-Emard (Centre de Biologie Nord, Hôpital de la Croix-Rousse, Lyon) for performing the assays of plasma catecholamines. This work was supported by a grant from the Association d'Aide aux Urémiques de Bretagne and from Region Bretagne.











