The role of food structure as key variable to achieve controlled nutrient digestion is a topic often explored in recent literature. According to different authors, regulated breakdown of macronutrients could be achieved by generation or preservation of structural barriers modulating enzymatic access to enclosed substrates( Reference Do, Singh and Oey 1 – Reference Pallares Pallares, Alvarez Miranda and Truong 4 ). In plant-based foods, major recommended components of a healthy and environmentally sustainable diet( Reference Willett, Rockström and Loken 5 ), macronutrients are naturally encapsulated by parenchyma cells. This microstructural assembling becomes majorly responsible for the relatively lower digestion rates during gastrointestinal digestion of structurally intact plant tissues( Reference Grundy, Edwards and Mackie 6 ). The (micro)structural architecture of foods and their associated particle sizes are the result of the series of processes they are subjected to before entering the gastrointestinal environment. Human mastication, for example, can be seen as a unit operation in which mechanical disintegration – and consequently structural modification – of food matrices takes place.
During oral processing of solid foods, two simultaneous processes occur: on the one hand, foods are converted into fragments small enough to be swallowed and, on the other hand, they get lubricated by saliva (which contains salivary α-amylase)( Reference Prinz and Lucas 7 – Reference Hiiemae 9 ). As a result of the mechanical disintegration of foods in the mouth, oral boluses with certain particle size distributions are generated( Reference Lovegrove, Edwards and De Noni 10 ). The particle size distribution (PSD) and (micro)structural architecture of a bolus are affected by the food type( Reference Hoebler, Karinthi and Devaux 8 , Reference Peyron, Mishellany and Woda 11 – Reference Mishellany, Woda and Labas 13 ) and its textural properties( Reference Hoebler, Devaux and Karinthi 14 ). Regarding the role of individual chewing behaviour, it has been reported that the PSD of ready-to-swallow boluses from different subjects are similar despite dissimilarities on physiological parameters (e.g. number of mastication cycles and mastication duration)( Reference Peyron, Mishellany and Woda 11 – Reference Mishellany, Woda and Labas 13 , Reference Grundy, Grassby and Mandalari 15 ).
Upon mastication of plant-based foods, two tissue failure modes can take place: cell rupture and cell separation( Reference Waldron, Parker and Smith 16 – Reference Van Buggenhout, Sila and Duvetter 18 ). The prevalence of one failure mode over the other is mainly governed by cell wall composition and the processing history of the food matrix prior to consumption( Reference Lovegrove, Edwards and De Noni 10 , Reference Capuano and Pellegrini 19 ). For instance, chewing of unprocessed foods such as fresh fruits and vegetables predominately results in cell breakage due to turgor pressure within and strong adhesive forces between cells( Reference Waldron, Parker and Smith 16 , Reference Capuano and Pellegrini 19 , Reference Lillford 20 ). On the contrary, cells of thermally treated plant-based foods are mostly separated upon mastication, a phenomenon that has been attributed to solubilisation of pectin in the middle lamella and the cell wall( Reference Chigwedere, Olaoye and Kyomugasho 21 – Reference Ribas-Agustí, Van Buggenhout and Palmero 26 ). Thermal processing is usually applied to increase the palatability of foods (e.g. by inducing softening), and its potential to tailor food digestion in situ by modification of the structural properties of foods at different length scales has been demonstrated( Reference Pallares Pallares, Rousseau and Chigwedere 27 ). Such potential is being currently explored for different nutrients, including starch. Starch that keeps on being (partially) encapsulated throughout passage in the gastrointestinal tract is considered positive since it allows (s)low glucose release in the small intestine and the delivery of resistant starch to the colon( Reference Do, Singh and Oey 1 , Reference Dhital, Bhattarai and Gorham 28 ).
In common beans (Phaseolus vulgaris L.), starch and protein are naturally encapsulated by the cell walls of cotyledon parenchyma cells( Reference Berrios, Swanson and Cheong 29 – Reference Hughes and Swanson 31 ). From a starch digestion point of view, these and other legumes (chickpeas, lentils, peas, etc.) seem to be remarkable candidates for providing modulated carbohydrate release, given that their microstructural assembling is kept after thermal processing, in vitro mechanical disintegration( Reference Pallares Pallares, Rousseau and Chigwedere 27 , Reference Dhital, Bhattarai and Gorham 28 , Reference Würsch, Del Vedovo and Koellreutter 32 – Reference Berg, Singh and Hardacre 34 ) and even human digestion( Reference Noah, Guillon and Bouchet 35 ). Common beans are usually soaked and thermally treated in water, after which they are subjected to mechanical degradation before or at the first step of human digestion. The duration of the thermal process is variable at both household and industrial levels and depends on the desired level of palatability, which is associated with a certain texture (i.e. hardness). Therefore, there is not a unique hardness, but rather a range of hardness values in which common beans can be perceived as palatable, depending on consumer preferences. As stated earlier, food textural properties will be a contributing factor for their PSD and microstructure after mastication( Reference Hoebler, Devaux and Karinthi 14 ), which subsequently will affect their digestive functionality. In the case of common beans, such textural properties are mainly determined by the intensity of the applied thermal processing. In this regard, we have recently reported the existence of differences on the in vitro starch digestion kinetics of cotyledon cells isolated from common beans after application of thermal treatments of varying duration( Reference Pallares Pallares, Alvarez Miranda and Truong 4 ). Nevertheless, it has not yet described the role of human mastication, as in vivo mechanical degradation step, with regard to the structural properties (i.e. PSD) of oral boluses from common beans with different process-induced hardness levels, nor its influence on the in vitro starch digestion kinetics of this food matrix.
The digestive functionality of foods will be highly dependent on the (micro)structural properties resulting after processing and mastication. Hence, the first objective of the present study was to assess the influence of process-induced common bean hardness on the PSD of in vivo generated oral boluses. The second objective was to evaluate the effect of in vivo induced common bean structural features on their in vitro starch digestion kinetics. Different human subjects were taken into consideration throughout the research, aiming to gather information on within- (as affected by hardness level) and between-subject (as affected by individual mastication behaviour) differences.
Materials and methods
Plant material
Food-grade dark red kidney beans (P. vulgaris L.), harvested and sun-dried in the USA in October 2015, were purchased from Casibeans©. Upon arrival at our facilities, the plant material was manually cleaned. The amount required for the mastication study was stored at ambient conditions for a maximum of 5 weeks.
Thermal processing of common beans
This experimental section was divided into two stages: first, the cooking profile of the studied plant material was determined by hardness measurement in order to identify its palatable range. Next, selected textural conditions were reproduced in a food-grade environment for sample preparation in the context of the mastication study.
Determination of the cooking profile
The cooking profile of the common beans used in the present study was obtained in a similar way as described in previous publications of our research unit( Reference Chigwedere, Olaoye and Kyomugasho 21 , Reference Pallares Pallares, Rousseau and Chigwedere 27 ). Briefly, common beans were soaked (about 25°C, 16 h) in excess demineralised water, after which the soaking media was discarded. In a subsequent step, they were thermally processed (95°C, f(t)) using demineralised water in excess. Thermal treatment time (0–180 min) was a process variable. An individual container was used for each processing time. Hardness was used to describe texture, and it was measured by means of a compression test (texture analyser TA.XT2i; Stable Micro Systems). Texture analysis after processing was standardised and performed after cooling down the processed beans to room temperature and isolating the individual cotyledons( Reference Pallares Pallares, Rousseau and Chigwedere 27 ). Average hardness values (n 20) were plotted against processing time in order to generate the cooking profile.
Using the cooking profile, distinct hardness values from the palatable range of common beans were chosen as relevant samples to be part of the mastication study. In addition, for the purpose of sample selection, finger pressing tests( Reference Vindiola 36 – Reference Kinyanjui, Njoroge and Makokha 38 ) and preliminary mastication experiments were performed.
Sample preparation for mastication study
Five hardness levels (associated with five specific processing times) were selected for the mastication study on the basis of distinctive but palatable textural properties obtained after cooking. Such hardness values were reproduced under food-grade conditions using thermal plates and cooking pots. The processing conditions were the same as before, that is, common beans were thermally treated at a temperature of 95°C, using water in excess as cooking media. Upon attainment of the desired processing time, the thermal treatment was stopped using an iced water bath and the cooking water was discarded. Processed beans were cooled down to room temperature before use in the mastication study. Although there was a waiting time between thermal treatment and mastication of common beans due to practical reasons, it was confirmed by preliminary experiments that this did not result in any induction of hardness deviations.
Given that the mastication study was divided into different sessions (as explained in the next section), each hardness level was generated on the particular scheduled session date. Following cooking, an amount of the generated sample was set aside for characterisation in terms of hardness (under identical conditions as depicted in the previous section), total starch content using the Megazyme Total Starch Assay kit (AA/AMG) and degree of starch gelatinisation by differential scanning calorimetry( Reference Pallares Pallares, Rousseau and Chigwedere 27 , Reference Salgado-Cruz, de la, Ramírez-Miranda and Díaz-Ramírez 39 ). Hardness determination was performed on the same day of cooking, before the corresponding mastication session. These two latter characterisations were done in subsequent analyses, after isolation (by removal of seed coats), mechanical disintegration (using mortar and pestle) and lyophilisation of cotyledons, as explained in our previous publications( Reference Pallares Pallares, Alvarez Miranda and Truong 4 , Reference Pallares Pallares, Rousseau and Chigwedere 27 ).
Mastication study
A mastication study divided into five sessions (one per each distinct but palatable hardness level selected) was executed. Participants were recruited from the staff and student group of the Laboratory of Food Technology (KU Leuven). Exclusion criteria comprised incomplete dentition (except unerupted third molars), any dental treatment at the moment of the mastication study or during the 3 months previous to it (unless it was a routine check-up), excessive tooth clenching and/or malocclusion, and presence of any infectious disease at the moment of the study. Once recruited, participants were informed in detail on the goals and methodology of the study before signing a written consent form. In total, twenty healthy adults participated in the study; however, four of them were excluded, given that they missed one or more sessions. Therefore, sixteen participants (eleven women and five men, mean age 26·1 (sem 1·0) years) were included in the final analysis.
The sessions of the mastication study were separated by at least 1 week (7 d), and the hardness levels under evaluation were randomised across the sessions. During each session, participants were asked to individually chew an amount of thermally processed common beans (approximately 100 g, which is the recommended amount in the newly proposed healthy reference diet( Reference Willett, Rockström and Loken 5 )), dividing it into mouthfuls. Each session consisted of training sequences (first three sequences) and main sequences (as many as required to finish the provided sample). In the first three sequences, participants were trained on the identification of their swallowing threshold by performing mastication exercises in which they were allowed to swallow the bolus after complete mastication( Reference Jalabert-Malbos, Mishellany-Dutour and Woda 12 ). Next, during the main sequences, participants had to chew the samples until their swallowing threshold, at which moment they expectorated the oral boluses into a plastic container. They were also asked to record the mastication duration (T) and the number of mastication cycles (N) for three of the main sequences. In this way, the mastication frequency (N/T) of each person per session was calculated from the averaged values. Participants were not informed of their masticatory performance during the mastication sessions. When they completed the mastication of the provided sample, participants were asked to rinse their mouth thoroughly. The rinsing water was expectorated into the plastic container containing the bolus. The collected boluses were frozen using liquid N2 and kept at −40°C until analysis.
The mastication study described in this section was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Social and Societal Ethics Committee of the KU Leuven (SMEC), Belgium (dossier no. G-2016 10 642). Written informed consent was obtained from all subjects.
Structural characterisation of boluses
Two different but complementary techniques were used for determining the PSD of the oral boluses collected during the mastication study: laser diffraction and wet sieving. This was done, as previously reported for other food matrices( Reference Peyron, Mishellany and Woda 11 , Reference Grundy, Grassby and Mandalari 15 ), with the aim of covering and characterising the entire range of particle sizes present after mastication of thermally treated common beans. As additional characterisation technique, the different constituent fractions of a randomly selected bolus were visually examined by taking photographic images.
Laser diffraction
Determination of PSD by laser diffraction was done as described in Pallares Pallares et al.( Reference Pallares Pallares, Alvarez Miranda and Truong 4 ). In short, each bolus was added into a stirred tank filled with demineralised water. Thereafter, the diluted sample was pumped into the measuring cell (pumping rate 30 %), where the laser light was scattered by the dispersed particles. The volumetric PSD were calculated based on the intensity profile of the scattered light, according to the Fraunhofer optical model using the software instrument.
For this analysis, particles larger than 2 mm were removed using a 2-mm mesh size sieve. This was done in order to avoid the obstruction of the particle size analyser (LS 13 320; Beckman Coulter Inc.) due to the presence of particle sizes above its upper size limit. Using this technique, the volume distribution was detected for particles between 0·04 and 2000 µm. More in detail, the PSD obtained by this technique included ninety-two particle sizes. Each collected bolus was analysed in triplicate to account for sampling variability, after which an average PSD was calculated.
Wet sieving
A random selection of expectorated boluses (n 5) from three process-induced hardness levels (hardest, intermediate and softest samples) was done. Each of the selected boluses was located on top of a stack of eight dried and weighed sieves, with the following apertures: 2·0, 1·0, 0·5, 0·25, 0·16, 0·125, 0·08 and 0·04 mm. A vibratory sieve shaker (AS 200; Retsch) equipped with a universal wet sieving clamping device was used for the separation of boluses into the respective fractions. The machine was programmed to operate with a vibratory amplitude of 2·5 mm for 4 min. During a first run, demineralised water was supplied from the top through a water spray system in order to aid particle separation. Next, a second run without water supply was performed in order to eliminate residual water. Following the sieving process, each sieve was thoroughly dried using paper and weighed again. In order to obtain the PSD, the fraction retained on each sieve was expressed as weight percentage relative to the mass of common beans before mastication.
In vitro simulated digestion of selected boluses
Specific boluses (previously wet sieved in the context of structural characterisation) were selected to be subjected to simulated digestion experiments, on the basis of having been generated by subjects with different masticatory parameters. Specifically, a subject in the upper limit and a subject in the lower limit of masticatory parameters were chosen. Digestion experiments were performed after thawing boluses in a water bath at 25°C. The quantity of sample used during the digestion experiments (1·25 g) was taken from thawed, well-mixed boluses.
The in vitro starch digestion kinetics of selected boluses (of which the moisture and total starch content were characterised) were determined using the harmonised INFOGEST in vitro digestion method( Reference Minekus, Alminger and Alvito 40 ). The conditions of the procedure were as depicted in our previous publication( Reference Pallares Pallares, Alvarez Miranda and Truong 4 ). Shortly, the gastric and small intestinal digestion phases were simulated in vitro. Throughout all the phases, temperature was kept at 37°C and constant mixing was provided. Appropriate electrolyte solutions, mimicking digestion fluids, were added in each simulated phase. Although no oral phase was considered, a dilution step consisting of mixing 1·25 g of bolus with simulated saliva (containing appropriate electrolytes but not salivary α-amylase) was initially performed. The reason for this dilution step was the fact that the saliva present in each bolus had been previously removed during wet sieving. Subsequently, simulated gastric juice containing pepsin enough to reach 2000 U/ml in the final mixture was added for simulation of the gastric phase for 2 h. Finally, the small intestinal phase was simulated by putting the in vitro gastric chime in contact with simulated intestinal juice containing pancreatin (about 200 U/ml (15 U/mg starch) in final digestion mixture, based on amylase activity), trypsin (enough to reach 100 U/ml in final digestion mixture), chymotrypsin (enough to reach 25 U/ml in final digestion mixture) and bile (10 mM in final mixture). This phase was simulated for 3 h, with withdrawal of samples as a function of digestion time. Samples were subjected to a heat shock for 5 min at 100°C in order to stop enzymatic activity, followed by centrifugation at 2000 g for 5 min in order to separate the supernatants from the remaining pellets. An individual tube was used for each digestion time studied during the in vitro small intestinal phase.
Supernatants were characterised in terms of starch digestion products; specifically, their reducing sugar content was determined in duplicate according to the dinitrosalicylic method( Reference Englyst and Hudson 41 ). For this purpose, 1 ml of (diluted) supernatant and 1 ml of colour reagent solution (made up of 3,5-dinitrosalicylic acid, potassium sodium tartrate tetrahydrate and 2 M NaOH) were mixed and incubated (oil bath ONE 10; Memmert) at 100°C for 15 min. The mixture was cooled down and diluted using 9 ml of Milli-Q water. The absorbance of the resultant coloured solution was measured at 540 nm in a spectrophotometer (Shimadzu UV-1800; Shimadzu). Reducing sugars were expressed as maltose reducing sugar equivalents using a maltose standard curve and then converted to starch equivalents using a conversion factor of 0·95. Finally, the average percentage of in vitro digested starch at each digestion time considered was calculated relative to the initial amount of starch on the boluses.
Kinetic modelling of in vitro starch digestion curves
In a similar way to our previous publication( Reference Pallares Pallares, Alvarez Miranda and Truong 4 ), an empirical logistic model (re-parametrised following the suggestions of Zwietering et al.( Reference Zwietering, Jongenburger and Rombouts 42 )) was chosen after a model discrimination procedure to describe the experimental kinetic data of in vitro starch digestion of in vivo generated common bean boluses:
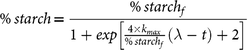 $$\%\,starch = {{\%\, starc{h_f}} \over {1 + exp\left[ {{{4 \times {k_{max}}} \over {\%\,starc{h_f}}}\left( {\lambda - t} \right) + 2} \right]}}$$
$$\%\,starch = {{\%\, starc{h_f}} \over {1 + exp\left[ {{{4 \times {k_{max}}} \over {\%\,starc{h_f}}}\left( {\lambda - t} \right) + 2} \right]}}$$
where % starch represents the amount of starch digested at digestion time t, % starchf is a plateau concentration at long digestion times, k max denotes the maximum reaction rate constant at the inflection point and λ symbolises the duration of the lag phase, for a given bolus. A simultaneous estimation of the three parameters of the model was done by nonlinear regression (SAS version 9.4; SAS Institute Inc.). The appropriateness of the model was evaluated by calculation of the R 2 adjusted and visual inspection of the parity and residual plots.
Statistical analysis
The number of participants recruited for our mastication study is similar to those for which statistically significant differences have been reported in previous evaluations of the structural properties (PSD) of oral boluses as affected by food type and mastication behaviour( Reference Peyron, Mishellany and Woda 11 , Reference Jalabert-Malbos, Mishellany-Dutour and Woda 12 , Reference Hoebler, Devaux and Karinthi 14 , Reference Grundy, Grassby and Mandalari 15 ). In the same way as reported by Grundy et al.( Reference Grundy, Grassby and Mandalari 15 ), a maximum dropout of 20 % was anticipated when selecting the number of participants for the chewing experiments.
Statistical analyses were performed using JMP statistical discovery software (JMP Pro 14; SAS Institute Inc.). All results are presented as means with their standard errors, unless otherwise stated. The presence of significant differences between hardness values of samples prepared for the mastication study and samples of the cooking profile having equivalent processing times was examined using two-sample t tests for unequal variances (two tailed, P<0·05). ANOVA was used to test for statistically significant differences among hardness values of the samples subjected to the mastication study as well as in masticatory parameters among hardness levels. When needed, post hoc analysis was done in order to examine each pairwise difference, using Tukey’s honestly significant difference test. Repeated-measures ANOVA was utilised to test the effect of process-induced hardness and participant on the PSD of oral boluses. Additionally, the PSD data were analysed using partial least square (PLS) regression, which was performed in Solo software (version 8.6, 2018; Eigenvector Research). A PLS model with three latent variables (LV) was selected to describe changes in PSD as a function of process-induced hardness (Y-variable). As selection criterion, if an LV explained more than 2 % of the Y-variance, it was included in the model. The output of the PLS model was visualised by construction of a biplot of the first two LV, using OriginPro 8 software (Origin Lab Corporation). Finally, for statistical comparison of the in vitro starch digestion kinetic profiles of the different boluses evaluated, the 95 % CI of their estimated model parameters were used.
Results
Selection of samples for mastication study based on cooking profile
Fig. 1 shows the cooking profile of the common beans used in the present study as well as their selected palatability range and the hardness values of the samples used for the mastication experiments (obtained by thermal processing under food-grade conditions). In addition, representative pictures of thermally treated common beans are shown in online Supplementary Fig. S1. As expected, the cooking profile was characterised by a hardness decrease as processing time increased. The palatability window, from which five samples were selected for the mastication study, ranged from approximately 12 500 g to 3500 g . Upon processing of the beans under food-grade conditions, similar hardness values at equivalent cooking times were obtained, with exception of the sample processed for 20 min (P=0·006).
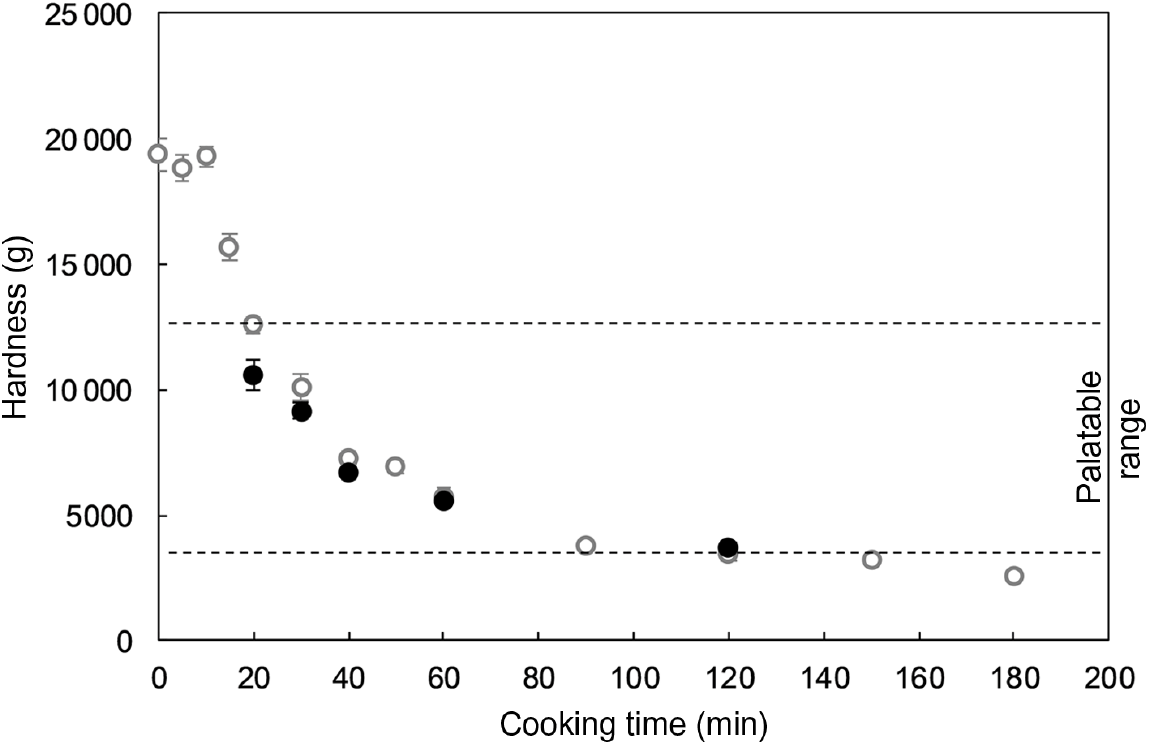
Fig. 1. Cooking profile of common beans (var. red kidney) at 95°C (○), including the palatable samples considered for the mastication study (•). An average hardness value is shown per each cooking time. Values are means (n 20), with standard errors represented by vertical bars. The same batch was used for generation of cooking profile and food-grade samples.
Samples used during the mastication study had statistically significant different hardness levels, except for those thermally processed for 40 and 60 min (P=0·186). Their total starch contents did not greatly differ, with an average value of 0·472±0·006 g of dry sample. Additionally, and in agreement with previous publications( Reference Pallares Pallares, Alvarez Miranda and Truong 4 , Reference Chigwedere, Olaoye and Kyomugasho 21 , Reference Dhital, Bhattarai and Gorham 28 , Reference Edwards, Warren and Campbell 43 ), the residual gelatinisation enthalpy was negligible in all cases (online Supplementary Fig. S2). The latter result implies that starch gelatinisation occurred at early stages during thermal treatment of common beans (less than 20 min was required for the process to be completed). Given that starch was completely gelatinised in all cases, observations made in subsequent analysis of in vitro starch digestion kinetics could be related to structural properties of the boluses obtained after mastication. Former investigations at our research unit have postulated that though cell wall hinders uncoiling and subsequent pasting of starch granules during thermal processing of common beans, it does not affect granular swelling or initial disruption of the native molecular order before pasting( Reference Chigwedere, Olaoye and Kyomugasho 21 ).
Masticatory parameters
The average values of the masticatory parameters obtained from the mastication study are shown in Table 1. In general, the number of mastication cycles and the sequence duration decreased as the hardness of the sample provided was reduced (i.e. intra-subject variability was detected as a result of different process-induced hardness levels). For both parameters, statistically significant differences were found between the softest sample (3697 g) and the two samples with highest hardness values (10 587 and 9154 g). In contrast, the mastication frequency was approximately the same for all the hardness levels and subjects evaluated (mean value 1·42 (sem 0·01)/s).
Table 1. Masticatory parameters for thermally processed common beans with different process-induced hardness levels
(Mean values with their standard errors; n 16)
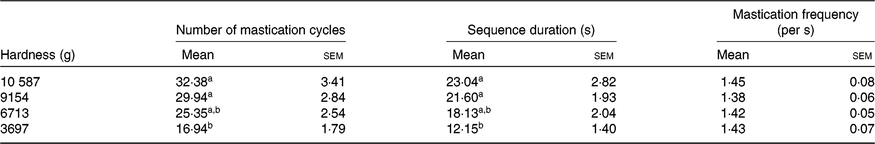
a,b Mean values within a column with unlike superscript letters are significantly different (P<0·05).
When assessing inter-subject differences in number of mastication cycles and sequence duration, it was observed that highly different individual mastication behaviours were present (as revealed by the standard deviation of the average values shown in online Supplementary Fig. S3). From hardest to softest sample, the number of mastication cycles varied between (lowest limit–upper limit) 12–66, 11–48, 10–42 and 6–34, respectively. A similar situation was observed in terms of sequence duration.
Structural properties of thermally processed and chewed common beans
The average PSD of oral boluses, as determined by laser diffraction and wet sieving, are shown in Figs. 2 and 3, respectively. Similar results were obtained regardless of the technique used. However, as stated earlier, particles with sizes > 2 mm were not detected using laser diffraction due to methodological reasons (upper size limit of the machine). As stated by other authors( Reference Grundy, Grassby and Mandalari 15 ), the size range that can be obtained during PSD measured by wet sieving is broader than the one obtained after laser diffraction analysis. Nonetheless, the latter technique allows better size resolution from a smaller amount of sample. These two complementary techniques enabled a complete characterisation of the PSD of the generated boluses.
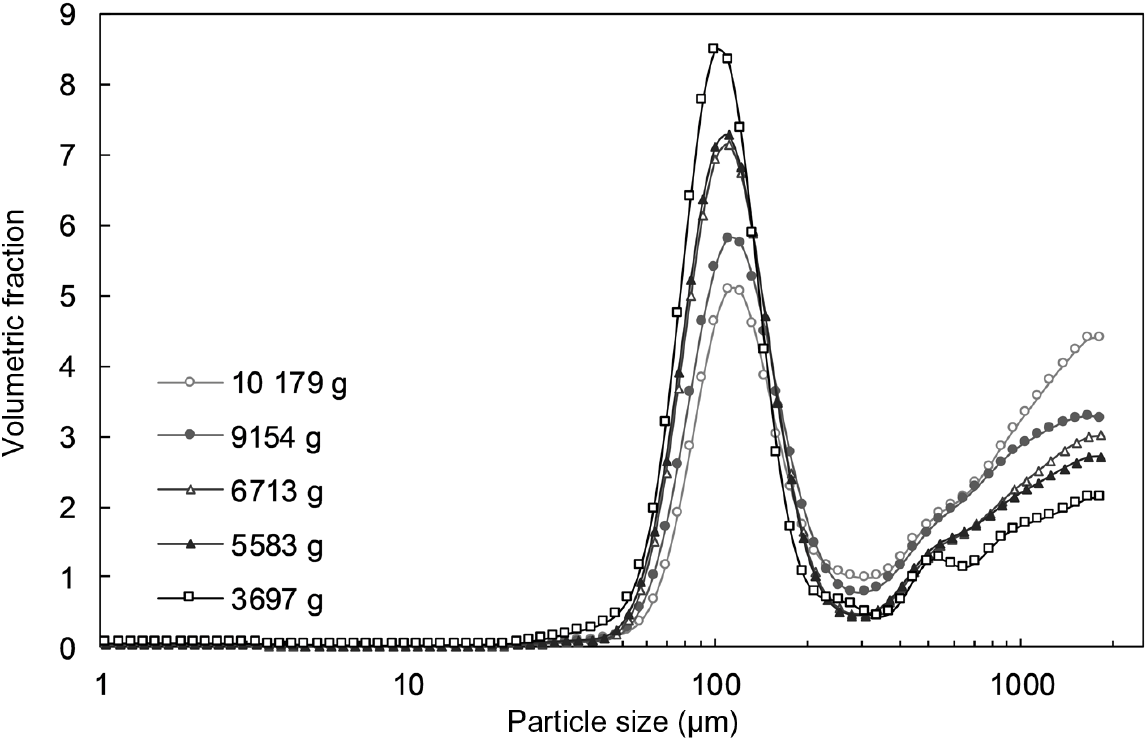
Fig. 2. Particle size distribution, as determined by laser diffraction, of oral boluses from thermally treated and in vivo masticated common beans with different hardness levels. Volumetric fraction (%) is plotted against size data (µm, on a log scale). An averaged curve is shown for each hardness level (n 16).
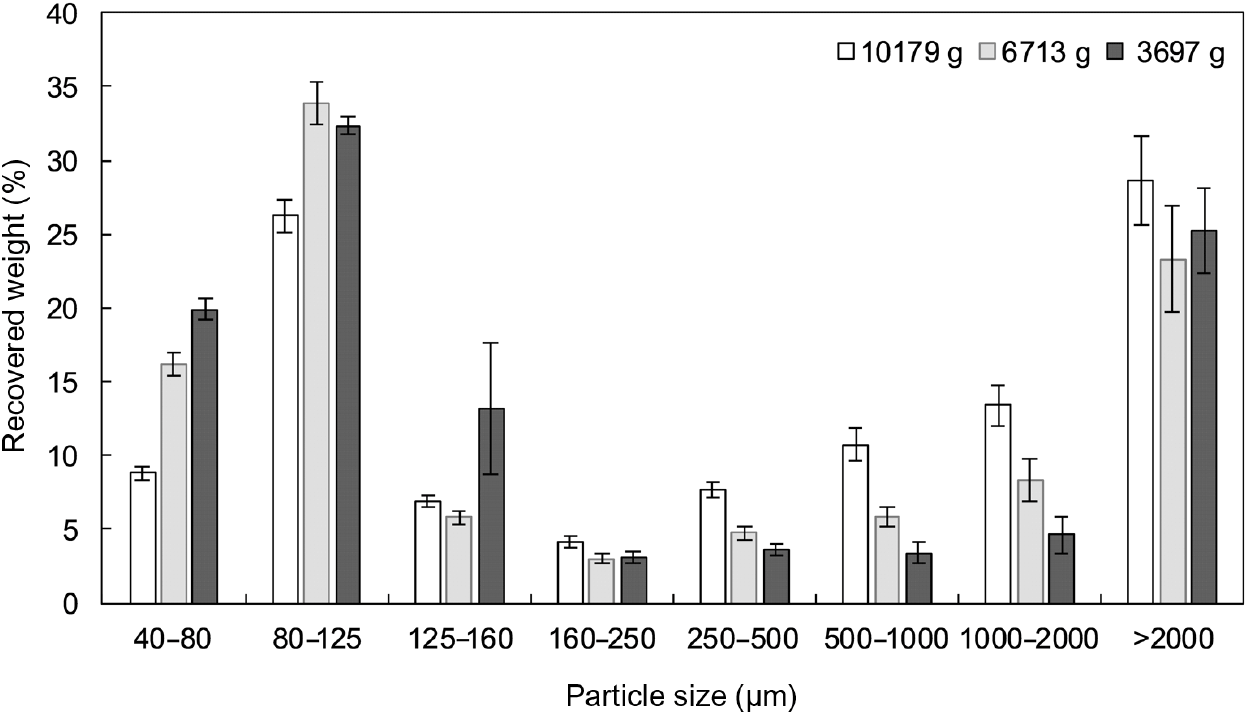
Fig. 3. Particle size distribution, as determined by wet sieving, of oral boluses from thermally treated and in vivo masticated common beans with different hardness levels. Weight recovered (%) is plotted against size data (µm). An averaged value per particle size range is shown for each hardness level. Values are means (n 5), with standard errors represented by vertical bars.
In all cases, PSD were multimodal and exhibited two major constituent fractions, irrespective of process-induced hardness level and/or mastication behaviour. The first characteristic fraction consisted of small particles, between 40 and 125 µm, whereas the second representative fraction was made up of big particles, with sizes larger than 2000 µm. Visual inspection of the separated fractions allowed to establish that the fraction of biggest particles mostly contained seed coat material, while fractions with sizes smaller than 2 mm were cotyledon-rich fractions (online Supplementary Fig. S4). These cotyledon-rich fractions could be differentiated at a microscopic level as individual cotyledon cells (40–125 µm), small cell clusters (125–250 µm) and big cell clusters (250–2000 µm) (online Supplementary Fig. S4).
Repeated-measures ANOVA of the PSD data obtained by laser diffraction (five process-induced hardness levels, sixteen subjects in each level) revealed that process-induced hardness had a statistically significant effect on the PSD of oral boluses (P<0·0001), while mastication behaviour did not (P=0·1141). A follow-up analysis with particle size as a factor showed that process-induced hardness had a significant effect on each of the ninety-two particle sizes detected by laser diffraction. PLS regression analysis of the same PSD data was also performed. The results of this analysis, that is, the change in particle size volumetric fraction as influenced by process-induced hardness level, are graphically shown in the biplot of Fig. 4. The first two LV of the PLS model explained 91 % of data variability. It can be seen in the obtained biplot that boluses obtained from common beans with the highest process-induced hardness are located on the right side, while boluses obtained from the softest samples are located on the left. Although within-group and between-group variations were observed in the samples, a more predominant effect of process-induced hardness level seemed to be present in agreement with the results of repeated-measures ANOVA. Regarding the variables evaluated (ninety-two particle sizes represented by open circles in Fig. 4), it was determined that those corresponding to individual cotyledon cells and big cell clusters were the ones contributing the most to data variability. This was established based on their position in the generated biplot, given that they were located far from the centre of the biplot and projected in the same or opposite direction as the Y-vector( Reference Buvé, Neckebroeck and Haenen 44 ). Certainly, the volumetric fraction of these particle size fractions changed the most as a function of process-induced hardness (Fig. 2). More in detail, the volumetric fraction of particle sizes located on the left side of the biplot (corresponding to individual cells) decreased with the increase in process-induced hardness, whereas the volumetric fraction of those on the right side (big cell clusters) increased.
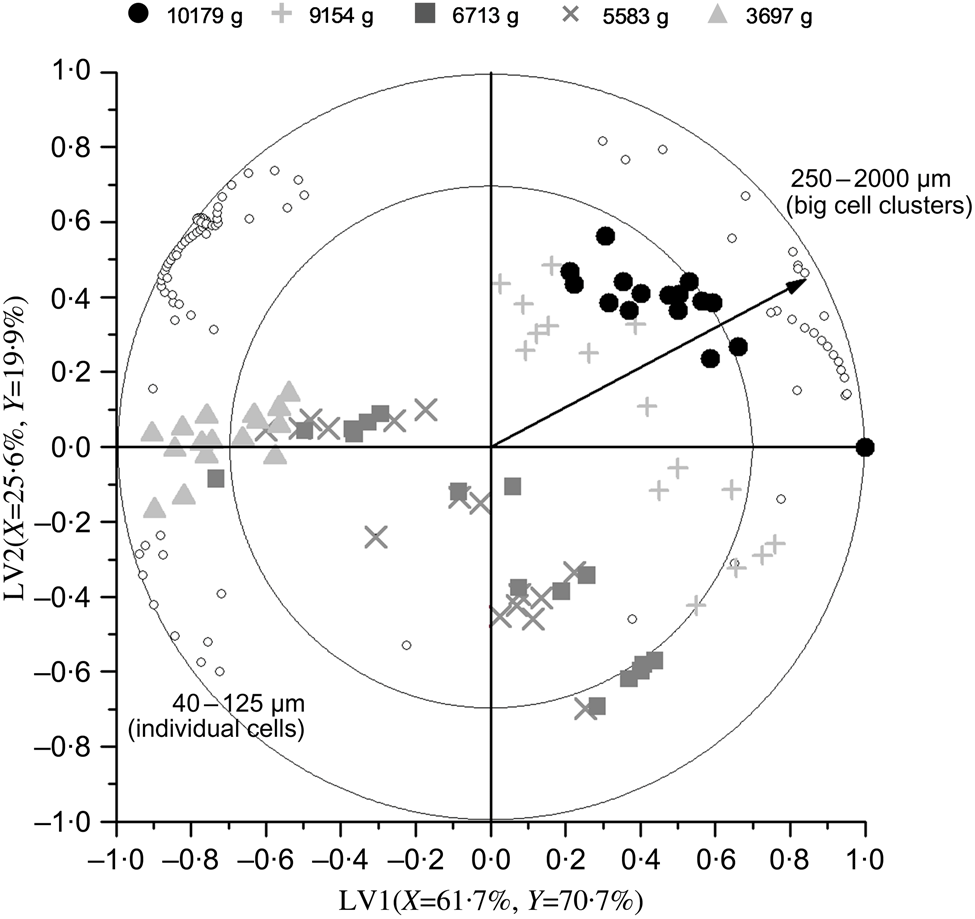
Fig. 4. Partial least square biplot describing the change in particle size distribution (determined by laser diffraction) as a function of process-induced hardness. The percentages of X- and Y-variance explained by the first two latent variables (LV) of the model are indicated on the respective axes. Particle size volumetric fractions are represented by open circles, while hardness levels are shown as different symbols. Correlation symbols of 100 % (outer circle) and 70 % (inner circle) were included to give an indication of the importance of each particle size in describing changes in bolus structure as a result of different process-induced hardness levels. The Y-vector represents the correlation loadings of process-induced hardness level (Y-variable).
Regarding the PSD obtained by wet sieving analysis, repeated-measures ANOVA with particle size as a factor displayed statistically significant differences among boluses from hardest (about 10 600 g), intermediate (about 6700 g) and softest (about 3700 g) common beans at all particle size ranges, with exception of 125–160 µm (P=0·1570), 160–250 µm (P=0·0811) and > 2000 µm (P=0·4871). From both analysis techniques (laser diffraction and wet sieving), it became clear that as process-induced hardness decreased, an increase in individual cotyledon cells occurred at the expense of a decrease in bigger cotyledon-rich fractions (more specifically: big cell clusters). On the contrary, and as expected, the fraction of seed coat material did not seem to be affected by hardness level.
In vitro digestion of selected boluses
The in vitro starch digestion kinetics of boluses from common beans with different process-induced hardness levels (each associated with a specific cooking time, i.e. 20, 40 and 120 min) were determined. For each hardness, boluses from two subjects with different masticatory parameters were selected. As stated earlier, a subject in the upper limit and a subject in the lower limit of masticatory parameters were chosen. On average, the number of mastication cycles and sequence duration of subject 1 were 2·6 times higher than for subject 2. It is important to mention that for this part of the experimental set-up, particles with sizes >2 mm were removed, since this fraction was mostly composed of seed coat material and was not significantly different among hardness levels (as reported in the previous section). The obtained results (% digested starch plotted against digestion time) are shown in Fig. 5.
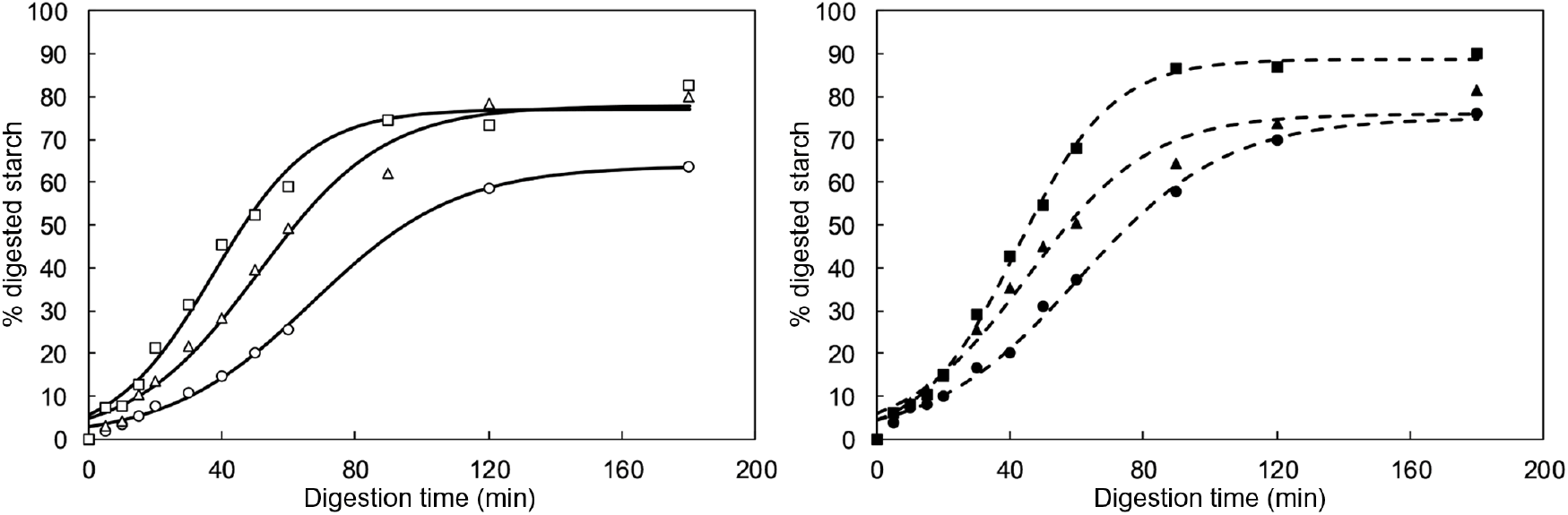
Fig. 5. In vitro starch digestion kinetic curves of oral boluses from thermally treated and in vivo masticated common beans with different hardness levels (circle=10 179 g; triangle=6713 g; square=3697 g). Experimental data points v. predicted values (using an empirical logistic model) are represented in the figure with symbols v. lines, respectively. Number of mastication cycles and sequence duration of subject 1 (left side, open symbols and full lines) were 2·6 times higher than for subject 2 (right side, closed symbols and dotted lines).
As expected, in vitro starch digestion in all boluses had an increasing trend until levelling off at a plateau value. This was the case regardless of hardness or subject. At early digestion times, a delay was observed in the release of starch digestion products, deviating the kinetic behaviour from a classical first-order type. In agreement with the results of structural characterisation, the in vitro starch digestion kinetics of different subjects did not seem to be prominently different, whereas process-induced hardness appeared to significantly influence the time-dependent behaviour of starch digestion. In order to quantitatively assess these observations, kinetic modelling of the data was done using an empirical, re-parametrised logistic model. The chosen equation is characterised by three parameters that allow to quantitatively describe each of the digestion phases observed experimentally( Reference Pallares Pallares, Alvarez Miranda and Truong 4 ), namely an initial lag phase, an intermediate dynamic phase and a final stationary phase. The calculated values of the kinetic model parameters and their 95 % CI are shown in Fig. 6 as a function of the cooking times associated with the process-induced hardness levels.
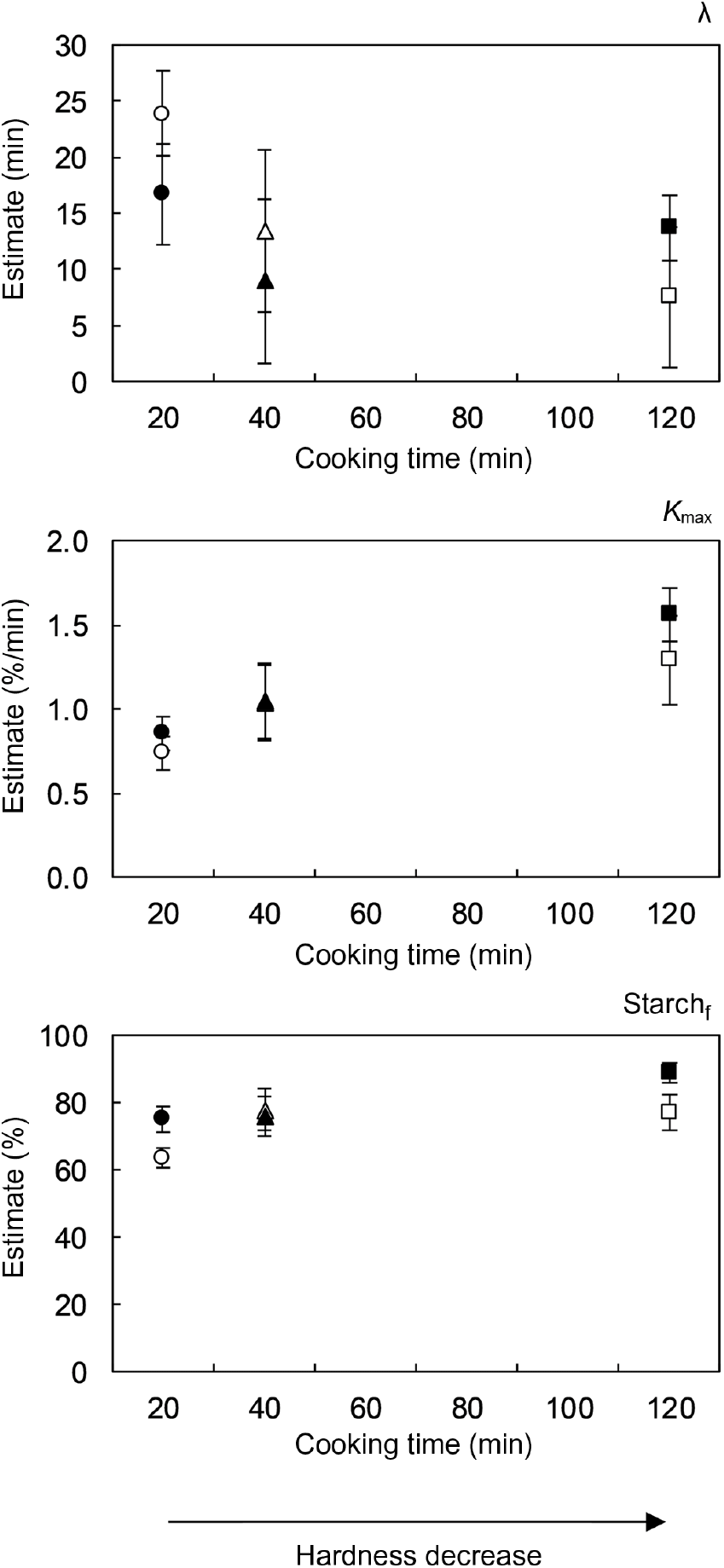
Fig. 6. The 95 % confidence intervals of the kinetic parameters of the empirical logistic model used to describe the in vitro starch digestion kinetics of oral boluses from thermally processed and in vivo masticated common beans. Results of two different subjects are shown. Number of mastication cycles and sequence duration of subject 1 (open symbols) were 2·6 times higher than for subject 2 (closed symbols).
Not surprisingly, the analysis of the 95 % CI of the estimated kinetic model parameters for each subject revealed the absence of statistically significant differences in most cases. Only the percentage of digested starch in boluses from the lowest and highest hardness levels was different among the evaluated subjects, though the calculated values were not strikingly dissimilar. In fact, this parameter did not seem to be affected by subject or hardness. On the contrary, a clear effect of process-induced hardness on the other two kinetic parameters of the model was observed. In a similar way as reported in our previous publication on the in vitro starch digestion of isolated common bean cotyledon cells( Reference Pallares Pallares, Alvarez Miranda and Truong 4 ), the duration of the lag phase (λ) decreased, while the maximum reaction rate constant at the inflection point (k max) increased with the decrease in hardness. Both kinetic parameters appeared to reach a final constant value at low hardness values. Statistically significant differences were found for the reaction rate constant of boluses generated from the hardest beans (about 10 600 g) and those obtained from softer ones (about 6700 and about 3700 g).
Discussion
The first research objective of this study was to gain insight into the PSD of in vivo generated oral boluses from common beans with different process-induced hardness levels. Specifically, we aimed to establish the predominant (micro)structural fractions after human mastication of common beans, and whether such properties were influenced by process-induced hardness level and/or mastication behaviour. For this purpose, a mastication study was conducted. Subjects participating in the study exhibited differences in masticatory parameters, though this was not reflected in the PSD of the collected boluses. This finding was in agreement with previous studies stating that PSD of different chewed foods depends on food type but not on individuals, despite variations in masticatory parameters between subjects( Reference Peyron, Mishellany and Woda 11 – Reference Mishellany, Woda and Labas 13 , Reference Grundy, Grassby and Mandalari 15 ). It has been previously reported that subjects adapt their mastication behaviour depending on factors such as physical food characteristics, including food texture( Reference Mishellany, Woda and Labas 13 , Reference Bonnet, Batisse and Peyron 45 ). Moreover, it has been postulated that a precisely determined bolus texture, which seems to depend on both particle size reduction and the creation of a cohesive bolus through lubrication with saliva, has to be achieved before swallowing occurs( Reference Peyron, Mishellany and Woda 11 , Reference Mishellany, Woda and Labas 13 , Reference Grundy, Grassby and Mandalari 15 ). We believe, in agreement to what has been stated by other authors, that the observed adaptation of individual masticatory behaviours to different process-induced hardness levels could be related to the generation of boluses with similar structural characteristics to be swallowed.
Unlike mastication behaviour, process-induced hardness did have a significant effect on the PSD of oral boluses from thermally processed common beans. Specifically, a higher amount of individual cotyledon cells was detected in boluses generated after mastication of softer beans. According to the PSD obtained, the increase in the fraction of individual cells was accompanied by a decrease in the fraction of big cell clusters. This could be explained by a higher pectin solubilisation in the middle lamella induced by longer processing times (associated with softer common beans), resulting in lower adhesion forces between cotyledon cells and higher separation upon application of mechanical forces( Reference Chigwedere, Olaoye and Kyomugasho 21 , Reference Pallares Pallares, Rousseau and Chigwedere 27 ). In other words, when harder beans were disintegrated, some big cell clusters remained due to the presence of stronger adhesive forces holding cells together. On the contrary, disintegration of softer beans mainly resulted in cell separation. Overall, oral boluses with similar PSD were generated by all subjects within a specific process-induced hardness level despite differences in masticatory parameters. Therefore, a significant effect of subject on the in vitro starch digestion kinetics was not anticipated.
Due to the presence of salivary α-amylase, mastication is also an enzymatic process in the context of starch digestion. Nevertheless, the contribution of this enzyme to the total carbohydrate breakdown is controversial due to, among others, its short contact time with the substrate as compared with the duration of the small intestinal phase (where pancreatic α-amylase is present)( Reference Lovegrove, Edwards and De Noni 10 , Reference Hoebler, Devaux and Karinthi 14 ). Recently, a relevant amylolytic salivary activity has been highlighted during simulation of an in vitro dynamic system with different acidification profiles, in an attempt to mimic the actual physiological conditions upon food ingestion( Reference Freitas, Le Feunteun and Panouillé 46 ). In the present study, the research question regarding nutritional functionality was related to the kinetics of starch digestion during in vitro simulation of the small intestinal digestive phase. Consequently, the percentage of digested starch was calculated relative to the amount of starch present in the boluses instead of the content in non-chewed samples. The total starch content of boluses, affected by enzymatic action of salivary α-amylase during mastication and the waiting time between expectoration and freezing of the bolus, was reduced by 0–20 % as compared with the amount present before mastication (data not shown). Based on the total starch content of each bolus, during in vitro simulated digestion, the amount of pancreatic amylase was adjusted to achieve an initial activity of 15 U/mg of starch present in the system.
The in vitro starch digestion kinetics of boluses generated from common beans with different hardness levels did not seem to be influenced by mastication behaviour. This is most likely due to the fact that, as discussed before, the PSD of boluses within a specific hardness level were not remarkably different despite variability on masticatory parameters. Thus, not surprisingly, samples sharing the same process intensity and exhibiting similar PSD showed similar nutritional functionality. Contrarily, an effect of process-induced hardness on starch digestion kinetics was present. Once more, this could be explained by considering the PSD of boluses among hardness levels. As process-induced hardness decreased, the amount of individual cells increased at the expense of a decrease in bigger starch-containing fractions (i.e. cell clusters). Therefore, the increase in surface area could have favoured the reaction rate, as it was indeed observed for samples with a higher amount of smaller particles. Nevertheless, despite an increase in surface area, the contact between amylases and starch was still affected by the presence of cell walls and proteins surrounding the carbohydrate. The most abundant starch-containing fraction of all boluses (with particle sizes between 40 and 125 µm) was characterised at the microstructural level as a fraction of individual closed cells . This is in agreement with our previous publications( Reference Pallares Pallares, Alvarez Miranda and Truong 4 , Reference Pallares Pallares, Rousseau and Chigwedere 27 ), as well as with publications of other authors( Reference Tovar, De Francisco and Bjorck 33 , Reference Berg, Singh and Hardacre 34 , Reference Rovalino-Córdova, Fogliano and Capuano 47 ). Such structural configuration possibly delayed the action of the enzyme on its substrate, as exemplified in the experimental data obtained by the presence of lag phases at early digestion times. A decreasing trend was identified in the kinetic parameter describing the duration of the lag phase as process-induced hardness decreased. As we suggested in our previous work( Reference Pallares Pallares, Alvarez Miranda and Truong 4 ), this is expected to be closely related to the status of the cell wall and protein matrix surrounding starch. These are the physical barriers through which pancreatic α-amylase has to diffuse before adsorption on and hydrolysis of starch granules. We have demonstrated previously that longer thermal processing times (i.e. those generating lower hardness levels) may induce higher cell wall permeability in common bean cotyledon cells, thus favouring shorter lag phases. Of course, the protein matrix constitutes an additional and important barrier contributing to the delayed amylase–starch encounter. In fact, both cell wall and protein matrix could have also contributed to the trend observed for the reaction rate constant of starch digestion (it increased as hardness decreased). A hypothesis we have put forward to explain the lower reaction rate constants in samples with lower cell wall permeability and/or higher amount of remaining protein after the gastric phase is as follows. On the one hand, it could be due to less enzyme diffusing inside the cellular space as a result of limited cell wall permeability. On the other hand, lower reaction rate constants could be a consequence of less enzyme being able to reach the substrate due to interaction with cell wall components and proteins( Reference Pallares Pallares, Alvarez Miranda and Truong 4 ). Differences in protein structural configuration, as induced by processing and/or digestion, could also be playing a role. Finally, a combination of all these factors is also plausible.
The in vitro starch digestion kinetics of the boluses evaluated in the present study highly resembles the behaviour we observed for individual cells isolated from thermally processed common beans( Reference Pallares Pallares, Alvarez Miranda and Truong 4 ). In fact, the estimated values of the kinetic parameters of individual cells and oral boluses obtained after application of similar processing intensities overlap. This is not astonishing, given the PSD of the boluses generated after human mastication (with predominant presence of individual cells, irrespective of process-induced hardness level or mastication behaviour). In summary, it can be stated that starch in common beans remains bio-encapsulated after thermal processing and in vivo mastication. Given the predominant presence of individual closed cells in the oral boluses, in vitro starch digestion kinetics seem to be mainly affected by process-induced changes in the starch-surrounding barriers (cell wall and/or protein matrix). These findings reinforce the importance of gaining knowledge on the barrier role of cell wall and eventually protein matrix in the context of starch digestion not only in common beans but in other pulses as well.
Overall, the nutritional functionality of thermally processed common beans is affected to a much greater extent by process-induced structural conditions than by mechanical degradation processes in the mouth (including effect of individual mastication behaviour). This constitutes an important finding in the context of product design for food scientists and technologists aiming to achieve tailored food digestion.
Acknowledgements
The authors would like to acknowledge Dr. ir. Carolien Buvé, expert in multivariate data analysis, for the help during PLS regression analysis and construction of biplots.
This work has been financially supported by the KU Leuven Research Fund. Additionally, this work is economically supported by the Louis Bonduelle Foundation through the ‘Louis Bonduelle Research Award’ (A. P. P., winner of the 2018 edition). The Louis Bonduelle Foundation had no role in the design, analysis or writing of this article.
The contributions of each author are as follows: A. P. P., M. H. and T. G. designed the study; A. P. P., B. L. and S. N. K. carried out the experimental work and analysed the samples; A. P. P. and T. G. analysed the data and A. P. P. wrote the manuscript, taking into account suggestions of T. G. All authors read and approved the final manuscript.
There is no conflict of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114519001624












