Article contents
Effect of probiotic supplementation on chemotherapy- and radiotherapy-related diarrhoea in patients with cancer: an umbrella review of systematic reviews and meta-analyses
Published online by Cambridge University Press: 18 April 2023
Abstract
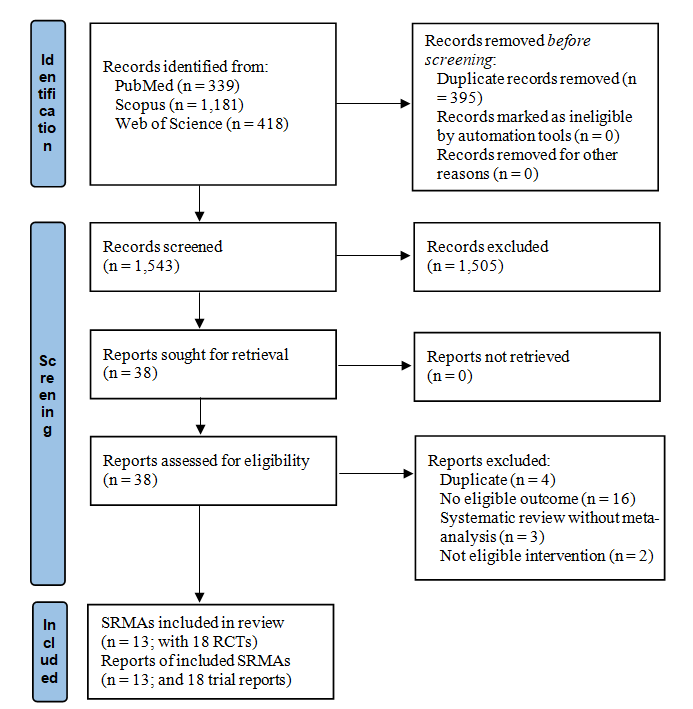
To date, several systematic reviews and meta-analyses (SRMA) have investigated the effects of probiotics, but the certainty of the evidence for an effect on chemotherapy and radiotherapy-related diarrhoea has not been assessed. We conducted an overview of SRMA, searching MEDLINE, Scopus, and ISI Web of Science from inception up to February 2022. We summarised the findings of eligible SRMA. Subsequently, we included randomised clinical trials (RCT) from the SRMA in meta-analyses, using a quality effects model to calculate the OR and 95 % CI for each outcome. We used ‘A Measurement Tool to Assess Systematic Reviews’ and the Cochrane risk of bias tool to assess the methodological quality of the SRMA and their RCT, respectively. We used the ‘Grading of Recommendations Assessment, Development, and Evaluation’.We included thirteen SRMA, which reported pooled effect sizes for chemotherapy and radiotherapy-related diarrhoea based on a total of eighteen RCT. Our meta-analyses demonstrated statistically significant beneficial effects from probiotics on all outcomes, except stool consistency; diarrhoea (any grade) OR 0·35 (95 % CI 0·22, 0·54), grade ≥ 2 diarrhoea 0·43 (0·25, 0·74), grade ≥ 3 diarrhoea 0·30 (0·15, 0·59), use of medication 0·49 (0·27, 0·88), soft stool 1·10 (0·44, 2·76) and watery stool 0·52 (0·29, 1·29). Probiotics use can reduce the incidence of diarrhoea in cancer patients in chemotherapy and radiotherapy, but the certainty of evidence for significant outcomes was very low and low.
- Type
- Systematic Review and Meta-Analysis
- Information
- Copyright
- © The Author(s), 2023. Published by Cambridge University Press on behalf of The Nutrition Society
References
- 1
- Cited by





