As glucose is the primary source of energy for the brain, it has been suggested that cognitive performance will be better supported over a longer time period following the consumption of low-GI foods (sustained energy delivery) than high-GI foods (rapid decline in energy)(1). However, when the effect of low-GI food consumption on cognitive performance is tested, study findings have been inconsistent(Reference Micha, Rogers and Nelson2–Reference Benton, Maconie and Williams9). Variability in study design and study limitations confounds interpretation of these inconsistent results. Study limitations include inadequate control of the test meal food type, physical size and macro- and micro-nutrient contents. Taken together, existing research indicates some evidence for a relationship between GI foods and cognitive performance, but replication of findings has been hampered due to several weaknesses in the research.
The hypothesis that GI affects cognitive performance is based on the knowledge that the brain solely relies on the metabolism of glucose for energy in its non-prolonged fasting state(Reference van de Ven, van der Graaf and Tack10). Overall, there is some evidence that a rapid rise in blood glucose concentration following a high-GI food may improve short-term memory during the first hour following a carbohydrate-containing food, whilst a more sustained glycaemic profile following ingestion of a low-GI food may be beneficial over a longer time frame, for example, throughout the morning(Reference Sunram-Lea and Owen11). The importance of breakfast on cognitive performance also has some support as there is evidence that breakfast consumption, as opposed to fasting and skipping breakfast, reduces cognitive decline throughout the morning(Reference Pollitt12–Reference Liu, Hwang and Dickerman14). One possibility is that circulating glucose levels following breakfast consumption (or lack of) influence cognitive performance. Although cognitive decline has been found to occur in an induced hypoglycaemic state(Reference Inkster and Frier15,Reference Graveling, Deary and Frier16) , non-diabetics do not typically experience hypoglycaemia due to the homoeostatic systems in place to prevent it(Reference Cryer, Nathan and Mulder17). Indeed, investigators have commented on the need for clarifying the effect of glycaemia on cognition and on whether cognitive function is affected by varying postprandial glycaemic responses to food(Reference Sunram-Lea and Owen11). Current evidence on this topic has predominantly emanated from studies that lack an appropriate control; consequently, results may be dependent upon qualities of the test foods other than the glycaemic response that they induce. The present research addresses prior limitations in the literature by controlling for differing food type, size and nutrient content of test foods by using a dessert that differs solely by speed of absorption of the carbohydrate sweetener. This was achieved through the use of sucrose (GI 65) for the higher-GI trifle and isomaltulose (GI 34) for the lower-GI trifle(18). Sucrose and isomaltulose molecules are both composed of one glucose and one fructose moiety but differ by their bond. The enzymatically rearranged α-1,6-glycosidic bond in isolmaltulose is more slowly digested and absorbed than the α-1,2-glycosidic bond in sucrose resulting in a lower glycaemic response. Both sugars are completely absorbed and provide the same amount of energy.
The objective of the present study is to determine whether GI independently influences cognitive performance in an adult population using a crossover study design that controls for all variables other than a difference in glycaemic response. The primary outcome was cognitive test scores tested at 60 and 120 min following consumption of the trifles. A lapse of 60 min allows for ingested carbohydrate to cross the blood–brain barrier(Reference Abi-Saab, Maggs and Jones19), while attention has been found to be better 2 h after ingesting a low-, compared with a high-GI food(Reference Ingwersen, Defeyter and Kennedy20). The secondary outcome was glycaemic response tested at baseline and at 60 and 120 min following consumption of the trifles.
Methods
Study design
This study is a double-blinded, randomised, controlled, crossover trial of cognitive function with repeated measures over time in relation to postprandial glycaemia after a sucrose- or isomaltulose-sweetened trifle. This study was conducted at the University of Otago in 2017. Approval for this study was granted by The University of Otago Human Ethics Committee (reference H17/011). The study has been registered with the Australian and New Zealand Clinical Trials Registry ACTRN12618001137280.
Eighty-three undergraduate students of human nutrition were invited to participate in this trial. The exclusion criteria were a diagnosis of diabetes; and colour-blindness would exclude participants from the cognitive test that required colour identification. Seventy-seven students provided informed written consent to participate. Participants were randomly scheduled to two sessions with an intervening 2- or 3-week gap. Participants were computer-randomised by a University staff member otherwise uninvolved in the study to the order in which they received each trifle using the RAND() function in Excel (Microsoft), stratified by sex. The investigators, participants and the biostatistician were blinded to treatment order with the code revealed only after the statistical analysis had been completed.
Test food
Trifle was chosen as the test food as it was considered: filling; easily alterable to suit special dietary needs and it would accommodate a large amount of sugar. The trifles differed only by the sweetener used, sucrose or isomaltulose. The trifles did not differ in nutrient content as tested and confirmed by Plant and Food Research, New Zealand (Table 1). The trifles were visually indistinguishable and served in identical containers. Equal numbers of both trifles were made by the study investigators ahead of each testing day and a University staff member otherwise uninvolved in the study coded the trifles by placing a coloured sticker on the containers. Participants who self-identified as vegan or gluten intolerant were served vegan jelly or custard and jelly, respectively, containing the same amount of sugar as the trifles. Two weeks prior to the cognitive test days, Glycaemic Index Otago determined the GI of the trifles (that contained 50 g of available carbohydrate) from the glycaemic response relative to a three times repeated glucose reference beverage in twelve subjects in accordance with the International Standard(21).
Table 1. Trifle nutrient information per serving

Testing day procedure
For standardisation, all participants were provided with breakfast cereal to eat at home on the morning of each test day. The breakfast cereals were given to the participants on the afternoon prior to each testing day. At the first occasion, each participant indicated the quantity he or she wanted to eat; this was weighed and placed in a sealable plastic bag. The same amount of cereal was provided prior to the second test day. Participants were free to add animal or plant milk to the cereal and were asked to use the same type of milk on each occasion. Participants chose his or her usual time for breakfast, they were asked to keep the time consistent on test days and to not eat anything else for breakfast. Participants were requested to fast after breakfast until they received their trifle at 12.00 hours. The trifle was ingested within 20 min accompanied with a 250 ml glass of water, and no other food or drink was permitted until the testing session had ended. Tests of cognition were performed at baseline and at 60 and 120 min after the participants first began eating the trifle. Participants were sitting on benches with a separation of at least 1 m. Study administrators invigilated the process, and the room was quiet during the administration of the cognitive tests to avoid audible distraction.
Cognitive tests
Six different cognitive tests were performed in the laboratories as described below. Each participant filled answers to the cognitive tests on test papers which were collected immediately after each individual test. Two projector screens in clear view of all participants were used to display test content, and audio content was played through surround speakers.
Five of the tests: free word recall, short delay word recall, long delay word recall, letter–number sequence and visuospatial recall were performed at baseline, and 60 and 120 min after the participants began eating the trifle. Reitan’s trail-making test part B was performed as the last test of the 60-min cognitive test battery. The content used in the tests on each testing day was different to ensure that no participant would be at an advantage if they heard about the tests from other participants before their own testing day.
Free word recall test
Word recall tests assess immediate and delayed verbal memory. The wordlists used in this study were adapted from Hopkins Verbal Learning Test which is standardised, validated and repeatable(Reference Brandt22). Hopkins wordlists contain twelve words to recall for each test with commonly used words grouped into three categories. The number of words in each list was increased to twenty to make the test more difficult as the list was repeated in each testing period. Audible versions of the wordlists enunciated by a computer-generated voice were played to participants at a frequency of one word/s. The words were listed in randomised order, and the order of words were altered each time the list was played ensuring the first and last word of the list were always different. To increase the cognitive demand of the free word recall test, tests were performed under the condition of divided attention(Reference Mulligan and Hartman23). To do this, participants performed motor sequences consisting of three hand actions, for example, OKAY-WAVE-DROP, while they listened to the wordlists. Different hand motor sequences for each free word tests were used so the distraction exercise was unfamiliar each time. The participants learned the motor sequence in successive order and reverse order, for example, LIFT-BACKWAVE-OKAY for 30 s prior to testing. Participants swapped between successive and reverse order after every five words played. While the wordlists were played aloud, the motor sequence description was displayed on the screens. Participants had 45 s to write down all of the words they could recall in no particular order immediately after the wordlist had played.
Short delay and long delay word recall test
At 5 min and 20 min after the completion of the free word recall test, participants had 45 s to write down as many of the words they could recall from that test.
Letter–number sequence recall test
Letter–number sequencing tests require participants to hold information in mind and mentally arrange that information(Reference Diamond24). Eight different sequences of letters and numbers were presented. The sequences increased in length by one letter or digit each time beginning from three characters long. The sequences were generated using the randomise function of Microsoft Excel (version 15.32). For consistency of difficulty, sequences were checked by the researchers to ensure that none of the sequences spelt any commonly known words, sounds or abbreviations, that sequences did not have the same digit adjacent to each another and that sequences with six or more characters followed a letter number pattern. Each sequence was displayed on screen for 5 s followed by a 10-s gap for participants to write their recollection of the sequence down.
Visuospatial recall test
The visuospatial test was designed by the researchers as repeatable tests were required that could be administered using a screen for display with answers on paper. For each test, a picture was displayed for 10 s followed by a blank screen for 3 s. All pictures contained thirty cartoon objects on a two-tone landscape background comprising mountains, buildings, trees, flowers, animals, a sun or moon and a person articulating a percentage in a speech bubble. Five questions were asked about the picture with each question being displayed for 20 s. The questions were either multiple choice or required the count of an object. Pictures were made using online software (Canva).
Reitan’s trail-making part B
The test measures visual attention, task switching and speed of processing(Reference Salthouse25,Reference Broshek, Barth and Groth-Marnat26) . The task involves joining dots containing letters and numbers in alternate, ascending order, for example, ‘1-A-2-B-3-C’ etc. without removing the pen off the paper until the final letter ‘L’. Performance is measured by the time it takes an individual to complete. The participants performed this test once at each session. To reduce learning effects, two versions of the trail-making test part B were used, the original and a mirror image of the original. To control for order, half of order 1 and order 2 were randomly assigned to perform the original test first followed by the mirror image. The other half were assigned to perform the tests in the reverse order.
Measurements
Measures of body weight and height were taken by a research assistant trained in anthropometry measurement. Body weight was measured using a Seca alpha 770 digital scale (Seca), accurate to 0·1 kg. Height was measured using a Holtain stadiometer (Holtain Limited), accurate to 0·01 cm. Using these measures, BMI was calculated by dividing body weight (kg) by the square of the height (m).
Blood collection and analysis
For the GI testing of the trifles in twelve participants, capillary blood samples were taken by fingerprick at baseline (immediately before trifle ingestion) and at 15, 30, 45, 60, 90 and 120 min thereafter. The trifle was eaten within 12 min of baseline. Measurement of blood glucose concentration was by a HemoCue glucose analyzer (HemoCue).
For all participants, 500 μl capillary blood samples were collected three times throughout each cognitive test session at baseline, and 60 and 120 min after participants had begun eating their trifles. Blood was collected into tubes containing 10 μl of potassium EDTA as an anticoagulant. Blood samples were centrifuged within 20 min of collection for 10 min at 2000 Relative Centrifugal Force. Plasma was pipetted into microcentrifuge tubes and stored at –80°C at the University of Otago, Human Nutrition Laboratories, New Zealand, for up to 1 month before analysis. Blood glucose concentrations were determined using an enzymatic colorimetric kit on a Cobas c 311 auto-analyser (Roche). The laboratory adheres to quality control procedures (Westgard rules) and the use of manufacturer’s controls. Intra-assay and inter-assay variations were determined with a pooled plasma sample. Repeatability and accuracy tests were performed using a Roche commercial control Precinorm U (two levels). CV were 1·25, 0·67 and 1·87 % for Precinorm U control one and two, and pooled plasma, respectively.
Statistical analysis
A sample of sixty-one was required to detect a difference of 0·5 standard deviations for all outcomes in a standardised form with 90 % power and α = 0·05, assuming a within-person correlation of at least 0·3. As this is a crossover trial, all participants were compared with themselves. Mixed-effects regression analysis was used to estimate the effects the sugars had on cognitive test scores at 60 and 120 min, with cognitive test scores as the outcome variable, treatment as the predictor variable and participant as a random effect. Analyses were adjusted for baseline score where relevant (no baseline for trail-making test) and for English as a second language, special diet and randomised order. Participants who were unable to finish their trifle were excluded. Residuals of all models were plotted and visually assessed for homogeneity of variance and normality. The mean difference with 95 % CI was calculated to estimate the difference in glycaemic response between treatments at 60 and 120 min on cognitive test days.
Results
Data from sixty-five participants were included in the analysis. The randomisation, allocation and exclusion of participants are given in Fig. 1. Participants who did not take part in both testing days or did not eat all of their trifle on the second testing day were excluded from the analysis. The demographic characteristics of participants are presented in Table 2. The study sample had a high ratio of females:males. Most participants were New Zealand European and in their early twenties. None of the participants had been diagnosed with diabetes. No one was colour blind, and so all complete visuospatial tests were used in the analysis.
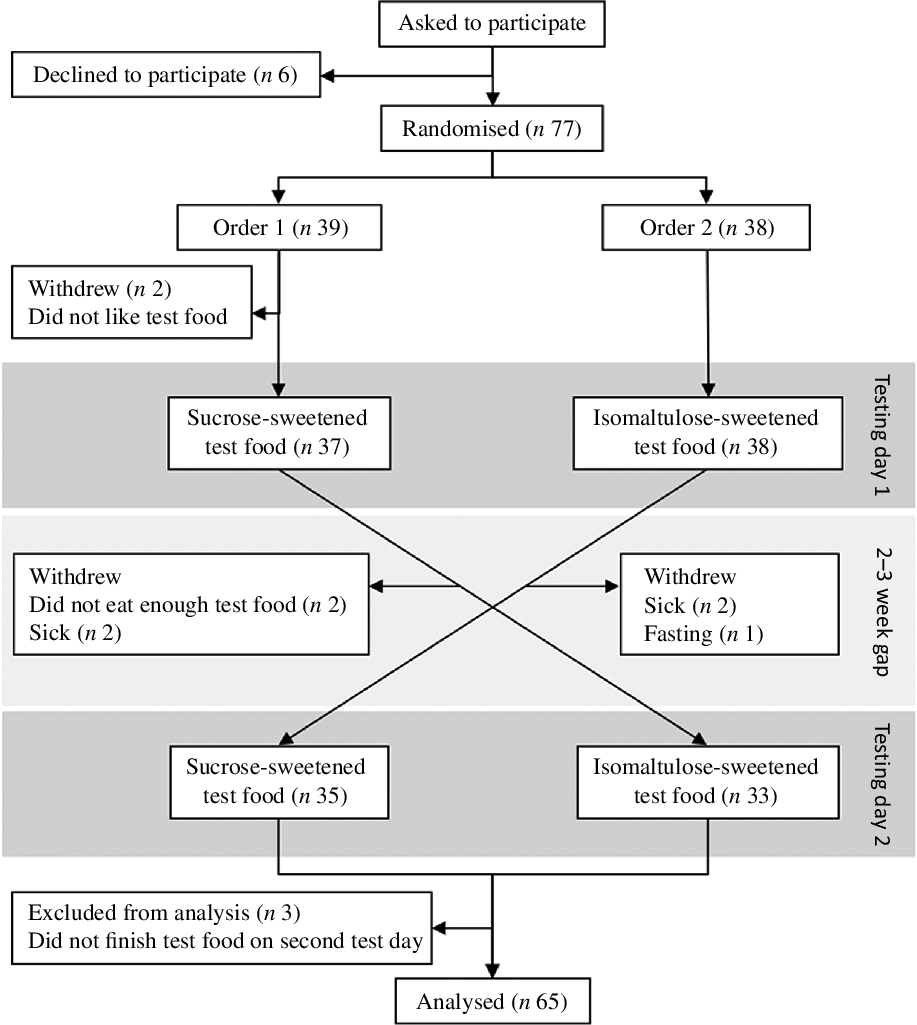
Fig. 1. Study design and participant flow diagram.
Table 2. Demographic characteristics of participants
(Mean values and standard deviations; medians and interquartile ranges (IQR); numbers and percentages)

The sucrose and isomaltulose trifles had GI of 44 and 33, respectively. The mean postprandial glycaemic response of the sixty-five participants to each trifle over the 2-h period is displayed in Fig. 2. During the cognitive tests, the plasma glucose concentration was 0·69 (95 % CI 0·31, 1·07) mmol/l lower at 60 min following ingestion of the isomaltulose compared with the sucrose-sweetened trifle. The mean between-trifle difference in plasma glucose concentration at 120 min was not statistically significantly different at 0·23 (95 % CI –0·06, 0·52) mmol/l.

Fig. 2. Glycaemic response curve to sucrose- and isomaltulose-sweetened trifles (n 65). * Statistically significantly different.  , Sucrose;
, Sucrose;  , isomaltulose.
, isomaltulose.
The effect of GI on cognition is reported in Table 3. No significant differences in cognitive function after sucrose- and isomaltulose-sweetened trifles were detected for any of the memory tests. On the trail-making test, participants performed nearly 8 s faster after the higher-GI sucrose trifle than after the lower-GI isomaltulose trifle (P = 0·037).
Table 3. Number of correct answers and adjusted mean differences at 60 and 120 min in cognitive test scores between sugars (with the exception of the trail-making test measured in seconds)*
(Mean values and standard deviations; mean differences and 95 % confidence intervals)

* Adjusted for English as a second language, special diet, baseline score (except for trail-making test) and order.
† No baseline test was undertaken, the values are mean and standard deviations of each group at 60 min.
Discussion
Although a difference in postprandial glycaemia was observed between the two trifles made with sugars of different GI, there was no difference in cognitive test scores for memory-related tests. The time to complete a trail-making test 1 h after trifle consumption was significantly shorter (better) following the higher-GI trifle.
The effect of postprandial glycaemia on cognitive test scores has been inconsistent, although among various tasks, the authors of a systematic review concluded that glycaemic manipulation was most consistently associated with memory(Reference Hoyland, Lawton and Dye27). Generating differences in glycaemic response by using a sipping strategy compared with a bolus, it was suggested that working memory was better supported by the sipping regimen, possibly due to the avoidance of a sharp decline in glycaemic response that had occurred following peak glucose concentration following bolus ingestion(Reference Nilsson, Radeborg and Bjorck28). However, the effect of the shape of the glycaemic response on cognition has been inconsistent. In accordance with the theory that a rapid decline in glycaemia may affect cognition, a better result for memory was found following a low- compared with a high-GI test beverage at a time when rapid glycaemic decline was occurring in people with good glucose tolerance(Reference Young and Benton5). In contrast, Benton et al. found no difference in memory following a period of more rapid decline (30–90 min) in glycaemia between low- and high-GI test foods(Reference Benton, Ruffin and Lassel29). A difference in the rate of glycaemic decline following test beverages was most evident in our sample between 30 and 60 min, with no difference in memory scores. The inconsistency in findings challenges the notion that memory is affected by fluctuations in postprandial glycaemic excursions. Indeed, cognitive performance was better after a high-fat compared with a high-carbohydrate meal, with the authors postulating that stable glucose and insulin concentrations were favourable to cognitive function over fluctuations in glycaemic metabolism(Reference Fischer, Colombani and Langhans30). There may be some support for this hypothesis. Memory test scores were not different following lower-, medium- and higher-glycaemic load breakfast meals when the tests were conducted between 1- and 3-h post-ingestion, at times of relative glycaemic stability(Reference Brindal, Baird and Danthiir31). However, Dye and colleagues conducted cognitive tests coinciding with major fluctuations and differences in glycaemia between test meals with no between-treatment differences found for working memory(Reference Dye, Gilsenan and Quadt6). These data are consistent with our findings; although our tests were conducted at a time when a difference in glycaemia was occurring, there was no difference in memory test scores. From our data, memory was independent of both postprandial fluctuations in glycaemia and of any differences in glycaemia between treatments. Thus, if memory is indeed the aspect of cognitive function most likely to be associated with glycaemia(Reference Hoyland, Lawton and Dye27), then it may be even more difficult to relate other aspects of cognitive functioning to postprandial glycaemia. This is consistent with the findings of a systematic review, in which the authors concluded that the body of evidence concerning glycaemia and cognitive functioning is inconclusive, recommending that future studies address methodological problems(Reference Philippou and Constantinou32).
The major methodological problems are with blinding and confounding. Some investigators have recognised this and designed studies to eliminate bias and to control well for confounding. Dye and colleagues used milk-based drinks sweetened with added sucrose or isomaltulose to generate differences in postprandial glycaemia with no consistent relationship found between glycaemia and cognitive test scores of immediate, delayed, recognition, verbal and working memory, and psychomotor performance(Reference Dye, Gilsenan and Quadt6). Young & Benton used glucose- or isomaltulose-sweetened breakfasts for children as crossover test meals and found no between-treatment difference in tests of speed of information, item memory at 1 h, spatial memory, reaction times or attention, with item memory at 3 h favouring the isomaltulose treatment(Reference Young and Benton5). The same authors undertook a study with a similar design in older participants partitioned into four data sets based on blood glucose characteristics following an oral glucose tolerance test(Reference Young and Benton4). The four data groups were: poorer glucose tolerance (≥7 mmol/l at 2 h) divided further into lowest blood glucose concentration that either did, or did not, fall below baseline; and similarly for people with better glucose tolerance (<7 mmol/l at 2 h) split according to the lowest blood glucose concentration. There was no difference in word memory scores between sucrose- and isomaltulose-sweetened breakfast at any of the three cognitive testing time points for three of the data sets comprising 129 of the participants. For the data set representing people with better glucose tolerance in whom blood glucose concentration remained above baseline (n 25), memory scores were better following the isomaltulose compared with the sucrose treatment; there was no effect of treatment in any of the data sets on tests of working memory, reaction time, semantic memory or vigilance(Reference Young and Benton4). Thus, the results of studies in which blinding and good control of confounding have been attained are variable but on the whole null. Young and Benton argued that an effect of glycaemia on memory may only be evident in people with good glycaemic control favouring a lower-glycaemic test meal, although contrary to this suggestion, people with poorer glucose tolerance had better memory following a high-glycaemic, glucose-sweetened meal. The authors were cautious about their findings, recommending that the study should be replicated before firm conclusions could be made(Reference Young and Benton4). This is an appropriate suggestion considering the effect that chance may have on the results of these sorts of trials in which a large number of comparisons are being made over a number of tests conducted at several time points. Indeed, our data are consistent with an overall null effect, including for memory, with a between-treatment difference found only for the trail-making test favouring the higher- glycaemic, sucrose-sweetened trifle. We did not adjust for multiple comparisons in the statistical analysis. Adjustment for multiple testing would not affect the estimates from the one ‘significant’ result that showed, on average, a 15 % difference in time to complete the trail-making test, which is a meaningful improvement. However, caution is needed in the interpretation of this outcome, as, to reduce learning effects, participants did not undertake a baseline trail-making test. Although each participant is compared with themselves, and the order of receiving each treatment was randomised, the fact that there is no baseline measure limits our ability to attribute the difference in performance in the trail-making test solely to the difference in sugars.
The major strengths of our study were a relatively large sample who were provided with test meals controlled for macronutrient and energy content, volume and appearance in a double-blind fashion with confirmed differences in glycaemia at the 1-h test point. A limitation was that the glycaemic differences attained between trifles were relatively small, with both trifles found to be low GI (<55). However, this was designed as a practical experiment in which the principle of replacing a higher-GI sugar (sucrose) with a lower-GI sugar (isomaltulose) was followed. Given this limitation, it may be of interest to repeat the study whilst optimising differences in glycaemia between test meals and beverages.
Acknowledgements
We gratefully acknowledge Ms Margi Bryant for her skills in blood sampling and Mr Ashley Duncan and Ms Michelle Harper for their expertise in blood processing and analysis.
The study was funded by the University of Otago.
B. J. V. conceived the study. All authors designed the study. O. M. M. and F. E. K. undertook the practical work. B. J. V. supervised the study. J. J. H. analysed the data. O. M. M. drafted the manuscript. All authors edited the manuscript.
The authors declare no conflicts of interest.










