Increased plasma total homocysteine (tHcy) has been reported to be associated with a variety of clinical outcomes, including hypertension, diabetes, chronic kidney diseases, CVD, cancer, psychiatric disorders and cognitive impairment( Reference Wald, Law and Morris 1 – Reference Xie, Yuan and Guo 3 ). It has been reported that hypertension and raised tHcy can synergistically increase the risk of stroke( Reference Towfighi, Markovic and Ovbiagele 4 , Reference Li, Jiang and Zhang 5 ). Furthermore, a significant positive dose–response association has been found between degree of tHcy reduction and systolic blood pressure (SBP) reduction( Reference Qin, Li and Sun 6 ), and decrease in CVD( Reference Li, Huang and Zheng 7 ) or carotid intima–media thickness( Reference Qin, Xu and Zhang 8 ). Consistently, a recent study found that the lowering of tHcy was significantly associated with a reduction in first stroke risk, and may possibly serve as a useful indicator for the use of folic acid therapy in the prevention of stroke( Reference Huang, Li and Li 9 ).
In previous randomised trials with long-term folic acid therapy, the percentage of tHcy reduction between groups ranged from 10 to 38 %( Reference Huo, Qin and Wang 10 – Reference Zhao, Wu and Li 12 ). Many of these previous trials were conducted in participants with CVD, and a high dose of folic acid (median: 2 mg) was used, often combined with vitamin B12 and/or B6 ( Reference Huo, Qin and Wang 10 – Reference Zhao, Wu and Li 12 ). In fact, a previous meta-analysis of randomised trials suggested that daily doses of 0·8 mg of folic acid had achieved the maximum reduction in serum tHcy levels( 13 ). Little further tHcy reduction is achieved by increasing the dose of folic acid above 0·8 mg/d( 13 ). Furthermore, two recent meta-analyses of randomised trials found that folic acid therapy significantly reduced the risk of stroke by more than 20 % among trials with a daily folic acid dosage of 0·8 mg or lower. Excessive doses of folic acid therapy did not lead to an additional beneficial effect( Reference Zhao, Wu and Li 12 ). More importantly, there has been a lack of randomised clinical trials that have comprehensively assessed the modifiers affecting the variation of tHcy reduction associated with long-term folic acid supplementation. The aim of the present study was to fill in these important gaps in knowledge regarding folic acid supplementation and the reduction of tHcy. A better understanding of the modifiable risk factors would be helpful for clinical and public health decision-making, for it would lead to early identification and effective risk reduction, and result in an individualised folic acid treatment regimen with the best benefit–risk ratio.
The main analyses of the China Stroke Primary Prevention Trial (CSPPT), which have been published previously, showed that folic acid therapy can reduce the risk of first stroke by 21 % among hypertensive patients( Reference Huo, Li and Qin 14 ). The present post hoc analysis of the CSPPT aimed to examine the potential modifiers in the association between long-term low-dose folic acid supplementation and the reduction of tHcy among hypertensive patients without major CVD, using data from the CSPPT.
Methods
Study participants
The methods and primary results of the CSPPT have been reported elsewhere( Reference Huo, Li and Qin 14 ). Briefly, the CSPPT was a multi-community, randomised, double-blind, controlled trial conducted from 19 May 2008 to 24 August 2013 in thirty-two communities in Jiangsu and Anhui provinces in China. Eligible participants were men and women aged 45–75 years old who had hypertension, defined as seated resting SBP ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg at both the screening and recruitment visit, or were on antihypertensive medication. The major exclusion criteria included history of physician-diagnosed stroke, myocardial infarction (MI), heart failure, post-coronary revascularisation or congenital heart disease.
The present study was approved by the ethics committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China (FWA assurance number FWA00001263), and was registered with ClinicalTrials.gov, NCT00794885. All participants gave written informed consent before data collection.
Intervention and follow-up
Eligible participants, stratified by methylenetetrahydrofolate reductase (MTHFR) C677T genotypes (CC, CT or TT), were randomly assigned, in a 1:1 ratio, to one of two treatment groups: a daily oral dose of one tablet containing 10 mg of enalapril and 0·8 mg of folic acid (single pill combination, the enalapril–folic acid group) or a daily oral dose of one tablet containing 10 mg of enalapril only (the enalapril group). All study investigators and participants were blinded to the randomisation procedure and the treatment assignments. During the trial period, concomitant use of other antihypertensive drugs (mainly Ca channel blockers (CCB) or diuretics) was allowed, but not B-vitamins.
Participants were followed up every 3 months. At each visit, participant blood pressure and pulse were measured, the number of pills taken between visits was counted and the use of concomitant medications and any adverse events were recorded.
Laboratory assays
Laboratory tests were performed at the core laboratory of the National Clinical Research Center for Kidney Disease (Nanfang Hospital, Guangzhou, China). Serum tHcy, fasting lipids and glucose were measured using automatic clinical analysers (Beckman Coulter). tHcy measurement was calibrated to the National Institute of Standards and Technology standard reference material SRM1955. MTHFR C677T polymorphisms were detected on an ABI PRISM® 7900HT sequence detection system (Life Technologies) using the TaqMan assay. Serum folate and B12 at both the baseline and the exit visit were measured by a commercial laboratory using a chemiluminescent immunoassay (New Industrial).
Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.
Major definitions
Hyperuricaemia was defined as a uric acid concentration ≥417 μmol/l (7 mg/dl) in men or ≥357 μmol/l (6 mg/dl) in women. A current smoker was defined as smoking at least one cigarette per d or smoking more than eighteen packs in the last year. Those who had smoked more than eighteen packs but did not smoke in the last year were defined as former smokers. We combined former smokers and current smokers into an ever-smoker group. Regular concomitant medication use was defined as taking the drug of interest for 180 or more cumulative days( Reference Xu, Qin and Li 15 ).
We defined metabolic vitamin B12 deficiency( Reference Robertson, Iemolo and Stabler 16 ) as a serum vitamin B12 level of <400 pmol/l (500 pg/ml) with normal renal function (≥60 ml/min per 1·73m2 ), a tHcy level of ≥14 μmol/l and a folate level of 10·4 ng/ml (75 % percentile) or more.
Statistical analysis
Means (standard deviations) and proportions were calculated for population characteristics by treatment group. Differences in population characteristics were compared using two-sample t tests, signed rank tests or χ 2 tests, accordingly.
The treatment effect, expressed as the difference in tHcy level at the exit visit minus that at baseline, was estimated using generalised linear regression models with adjustment for age, sex, study centre, use of angiotensin-converting enzyme inhibitors (ACEI), MTHFR C677T genotypes, smoking status, BMI, tHcy, fasting glucose, total cholesterol, eGFR, folate, vitamin B12, uric acid and SBP at baseline, as well as time-averaged SBP, concomitant use of CCB and concomitant use of diuretics during the trial period. In additional exploratory analyses, possible modifications of the treatment effects on the primary outcome were assessed for the variables, including age (<60 v. ≥60 years), sex, MTHFR C677T genotypes, smoking (never v. ever smoking), hyperuricaemia (yes v. no), folate (<8·0 (median) v. ≥8·0 ng/ml), tHcy (<12·5 (median), ≥12·5 μmol/l), vitamin B12 (<380 (median) v. ≥380 pg/ml), eGFR (<60, 60–<90 and ≥90 ml/min per 1·73 m2), SBP at baseline (<160 v. ≥160 mmHg), as well as time-averaged SBP (<140 v. ≥140 mmHg) and concomitant use of diuretics (yes v. no) during the trial period.
A two-tailed P<0·05 was considered statistically significant in all analyses. R software, version 3.3.1 (http://www.R-project.org/), was used for all statistical analyses.
Results
Study participants and baseline characteristics
As illustrated in the flow chart (online Supplementary Fig. S1), a total of 20 702 participants were enrolled and randomised in the CSPPT. Of those, 10 348 participants were assigned to the enalapril–folic acid group and 10 354 participants to the enalapril group. We excluded participants who had missing data on tHcy measurements at the baseline (n 278) or exit visit (n 3557), leaving 16 867 (n 8418 in the enalapril–folic acid group and n 8449 in the enalapril group) participants in the final analysis. Participants not included in the analyses did not differ in baseline characteristics from those included in the analyses (online Supplementary Table S1).
All baseline characteristics were comparable between the enalapril and enalapril–folic acid groups (Table 1). There were 1572 (9·3 %) participants undergoing treatment with ACEI at baseline.
Table 1 Baseline characteristics of the China Stroke Primary Prevention Trial study participantsFootnote * (Numbers and percentages; mean values and standard deviations; medians and 25th–75th percentiles)
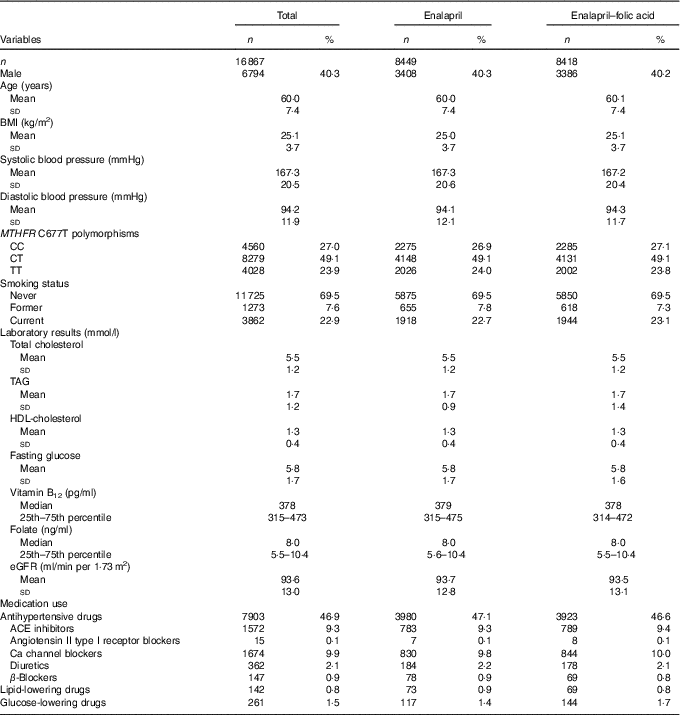
MTHFR, methylenetetrahydrofolate reductase; eGFR, estimated glomerular filtration rate; ACE, angiotensin-converting enzyme.
* For continuous variables, values are presented as means and standard deviations.
Blood pressure at baseline and during the treatment period
Mean SBP and DBP were highly comparable between the two groups at baseline and over the course of the trial (online Supplementary Tables S2 and S3).
Concomitant medications
The most common concomitant medications were CCB and diuretics, which were used by 82 and 53 % of the participants, respectively. There were no significant differences in concomitant medication use during the trial between the two treatment groups. The use of lipid-lowering, glucose-lowering and anti-platelet medications were very rare, all at a frequency of <2 % (online Supplementary Table S4).
Effect of folic acid therapy on change in total homocysteine levels
Mean baseline tHcy levels were comparable between the two groups (enalapril group, 14·5 (sd 8·5) μmol/l; enalapril–folic acid group, 14·4 (sd 8·1) μmol/l). After a median treatment period of 4·5 years, mean tHcy was 14·6 (sd 8·0) μmol/l in the enalapril group compared with 12·9 (sd 6·3) μmol/l in the enalapril–folic acid group (group difference: −1·6 μmol/l; 95 % CI −1·8, −1·4 μmol/l).
As expected, a greater group difference was found in participants with higher baseline tHcy levels (≥12·5 (median): −2·4; 95 % CI −2·9, −2·0; v. <12·5 μmol/l: −0·7; 95 % CI −0·9, −0·6 μmol/l) (Table 2).
Table 2 Mean total homocysteine levels (μmol/l) at baseline and exit visit by treatment group (Mean values and standard deviations; mean values and 95 % confidence intervals)
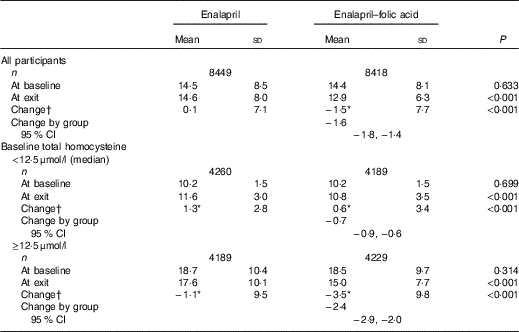
* Significantly different from baseline (P<0·05).
† Change=total homocysteine level at exit−total homocysteine level at baseline.
Furthermore, although the group differences were all significant in participants with lower (<12·5 μmol/l) or higher (≥12·5 μmol/l) baseline tHcy levels, tHcy increased in those with baseline tHcy <12·5 μmol/l, whereas it decreased in those with baseline tHcy ≥12·5 μmol/l in both treatment groups.
Stratified analyses by potential effect modifiers
Stratified analyses were performed to assess the treatment effect on the primary outcome in various subgroups (Fig. 1). After adjustment for baseline tHcy and other important covariates (age, sex, study centre, use of ACEI, MTHFR C677T genotypes, smoking status, BMI, fasting glucose, total cholesterol, eGFR, folate, vitamin B12, uric acid and SBP at baseline, as well as time-averaged SBP, concomitant use of CCB and concomitant use of diuretics during the trial period), a greater degree of tHcy reduction was observed in certain subgroups: males, the MTHFR 677TT genotype, higher baseline tHcy levels, lower folate levels, eGFR <60 ml/min per 1·73 m2, concomitant use of diuretics and ever smokers (P for all interactions <0·05). However, SBP either at baseline or over the treatment period, baseline use of ACEI and concomitant use of CCB had no significant effect on the efficacy of folic acid therapy in lowering tHcy levels (Fig. 1 and online Supplementary Table S5).
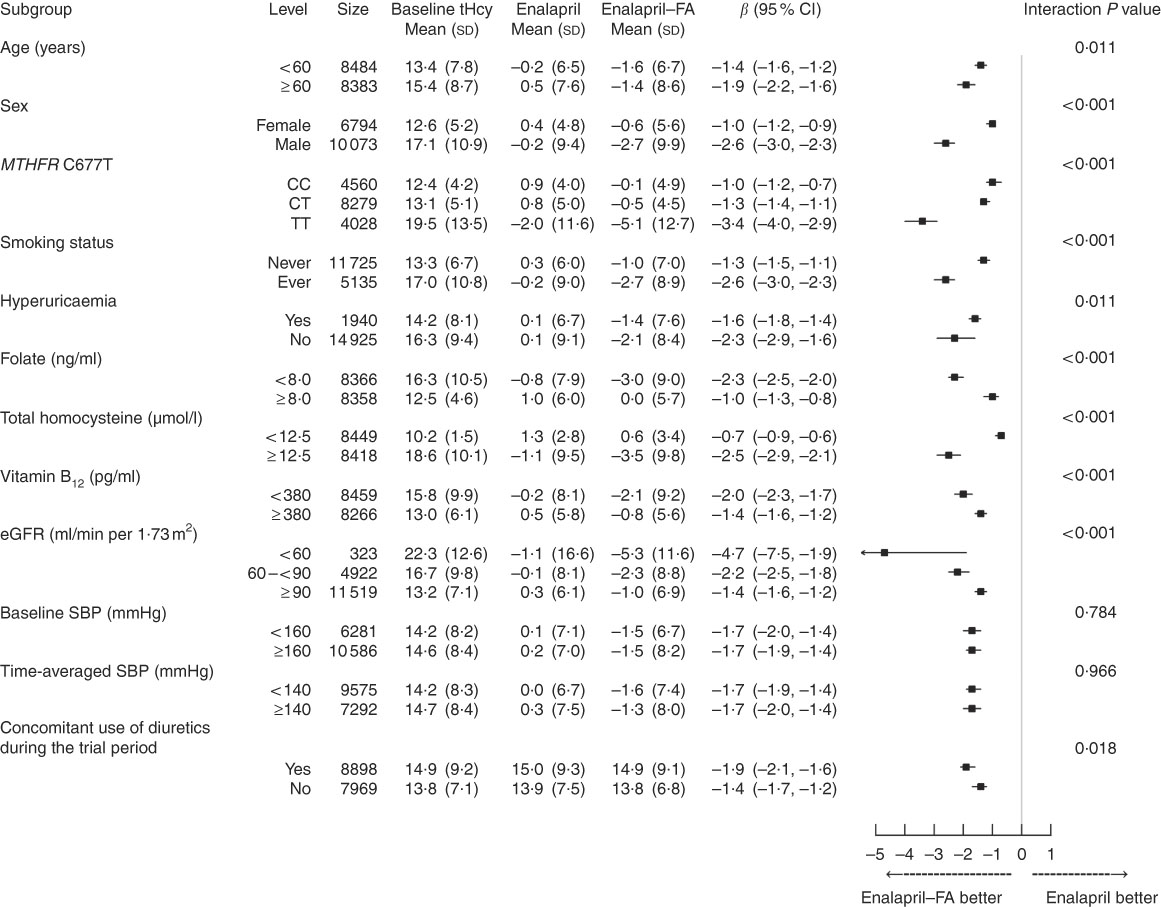
Fig. 1 Stratified analyses by potential effect modifiers. Adjusted, if not stratified, for age, sex, study centre, use of angiotensin-converting enzyme inhibitors, methylenetetrahydrofolate reductase (MTHFR) C677T genotypes, smoking status, BMI, total homocysteine (tHcy), fasting glucose, total cholesterol, estimated glomerular filtration rate (eGFR), folate, vitamin B12, uric acid and systolic blood pressure (SBP) at baseline, as well as time-averaged SBP, concomitant use of calcium channel blockers and concomitant use of diuretics during the trial period. Hyperuricaemia was defined as a uric acid concentration ≥417 μmol/l (7 mg/dl) in men or ≥357 μmol/l (6 mg/dl) in women. FA, folic acid.
When split into two groups according to baseline tHcy, similar trends for the same modifiers were found in participants with higher (≥12·5 μmol/l) (Fig. 2) or lower (<12·5 μmol/l) (Fig. 3) tHcy levels.
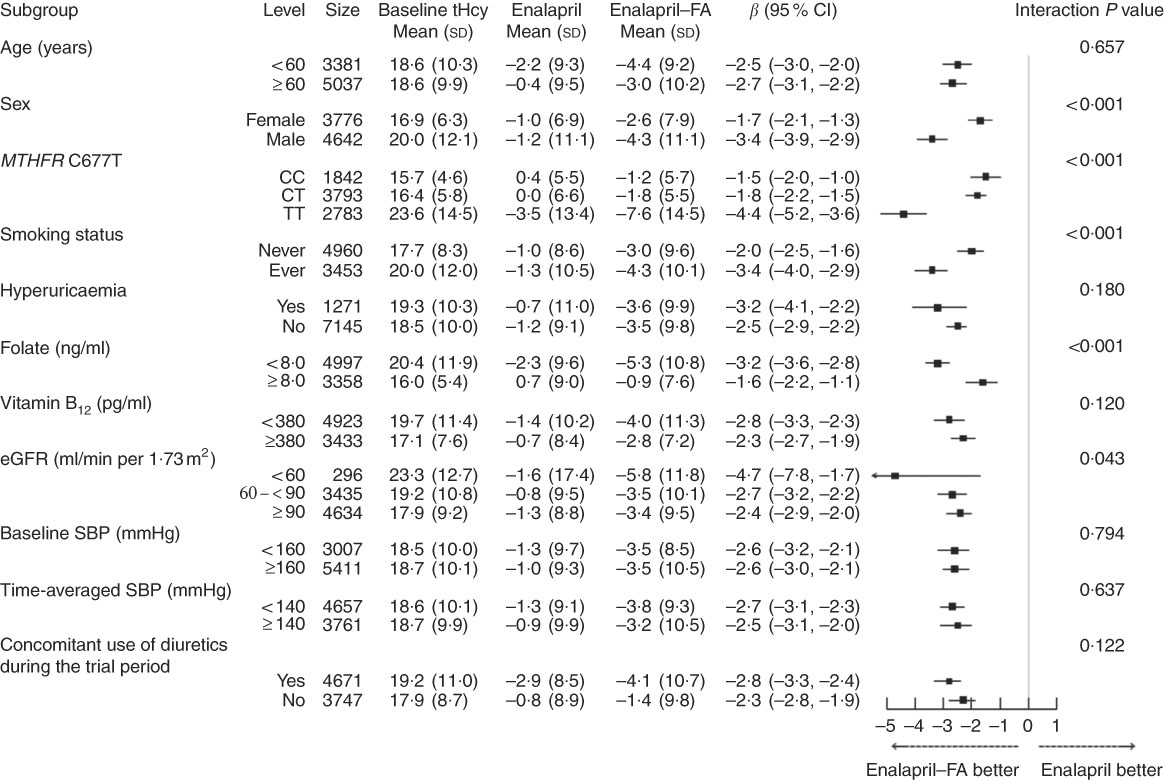
Fig. 2 Stratified analyses by potential effect modifiers in patients with higher baseline total homocysteine (tHcy) levels (≥12·5 μmol/l). Adjusted, if not stratified, for age, sex, study centre, use of angiotensin-converting enzyme inhibitors, methylenetetrahydrofolate reductase (MTHFR) C677T genotypes, smoking status, BMI, tHcy, fasting glucose, total cholesterol, estimated glomerular filtration rate (eGFR), folate, vitamin B12, uric acid and systolic blood pressure (SBP) at baseline, as well as time-averaged SBP, concomitant use of calcium channel blockers and concomitant use of diuretics during the trial period. Hyperuricaemia was defined as a uric acid concentration ≥417 μmol/l (7 mg/dl) in men or ≥357 μmol/l (6 mg/dl) in women. FA, folic acid.
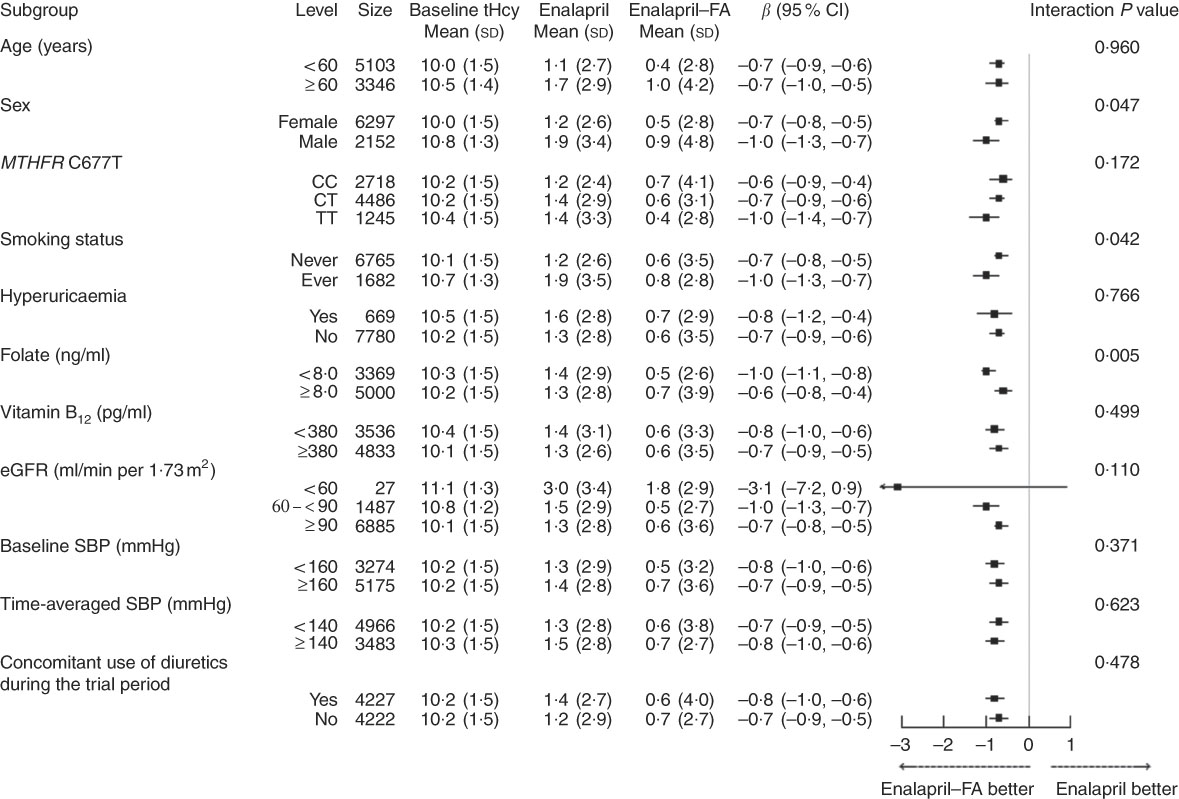
Fig. 3 Stratified analyses by potential effect modifiers in patients with lower baseline total homocysteine (tHcy) levels (<12·5 μmol/l). Adjusted, if not stratified, for age, sex, study centre, use of angiotensin-converting enzyme inhibitors, methylenetetrahydrofolate reductase (MTHFR) C677T genotypes, smoking status, BMI, tHcy, fasting glucose, total cholesterol, estimated glomerular filtration rate (eGFR), folate, vitamin B12, uric acid and systolic blood pressure (SBP) at baseline, as well as time-averaged SBP, concomitant use of calcium channel blockers and concomitant use of diuretics during the trial period. Hyperuricaemia was defined as a uric acid concentration ≥417 μmol/l (7 mg/dl) in men or ≥357 μmol/l (6 mg/dl) in women. FA, folic acid.
The prevalence of metabolic B12 deficiency was about 4·0 %. The tHcy-lowering effect of folic acid therapy was attenuated and became insignificant in participants with metabolic B12 deficiency (online Supplementary Table S6).
Discussion
Our present analysis is the first study to comprehensively evaluate the effect modifiers of long-term daily 0·8-mg folic acid supplementation in lowering tHcy levels among adults with hypertension without stroke and MI. As expected, long-term daily 0·8-mg folic acid supplementation significantly lowered tHcy levels in this population. More importantly, male, MTHFR 677TT genotype, higher baseline tHcy levels, lower folate levels, eGFR <60 ml/min per 1·73 m2, ever smokers and concomitant use of diuretics were major modifiers for the efficacy of folic acid supplementation in lowering tHcy levels. All these factors should be taken into account in the identification of participants who may be most likely to benefit from tHcy-lowering therapy.
Serum tHcy concentration is determined by genetic and a series of environmental factors. MTHFR is a critical enzyme for folate and tHcy metabolism. MTH FR converts 5,10-methylenetetrahydrofolate into 5-methyltetrahydrofolate, which is required for the remethylation of tHcy. The polymorphism of MTHFR 677C→T leads to a reduction in enzyme activity, resulting in increased concentrations of tHcy and lower levels of serum folate( Reference Stanger, Herrmann and Pietrzik 2 ). Serum tHcy increases with age, and men usually have higher levels than women with the same age. The sex-related tHcy difference may be partly explained by the effect of oestrogen in women( Reference Refsum, Smith and Ueland 17 ). Furthermore, patients with chronic kidney diseases have lower levels of folate owing to inadequate intake, increased loss or demand, and usually have higher tHcy levels. At the same time, tobacco smoke exposure is associated with higher tHcy levels owing to decreased folate intake( Reference Mathews, Yudkin and Smith 18 , Reference Ortega, Requejo and Lopez-Sobaler 19 ) and increased folate turnover( Reference Yanbaeva, Dentener and Creutzberg 20 , Reference Ulvik, Ebbing and Hustad 21 ). Accordingly, our study also suggests that participants with the MTHFR 677 TT genotype, lower folate or eGFR levels and those who are males and ever smokers seemed to have higher tHcy levels at baseline (Fig. 1). However, it is still unknown whether these factors modify the effect of long-term folic acid supplementation in reducing tHcy.
Consistent with previous studies( 13 , 22 ), baseline tHcy concentration was the major determinant of tHcy-lowering after folic acid treatment in the present study. A greater tHcy reduction was found in participants with higher baseline tHcy levels. More importantly, although the group difference was also significant among participants with lower baseline tHcy levels, tHcy levels significantly increased even among those in the folic acid treatment group over the 4·5-year treatment period. These results suggest that, beyond folic acid therapy, more safe and effective strategies need to be developed for the further control of tHcy levels.
After the adjustment of baseline tHcy, MTHFR C677T did not significantly affect the tHcy-lowering effect of folic acid therapy in our previous study (n 445)( Reference Qin, Li and Cui 23 ), nor in the study by Ho et al.( Reference Ho, Eikelboom and Hankey 24 ) (n 443). However, the study by Fohr et al.( Reference Fohr, Prinz-Langenohl and Bronstrup 25 ) suggested that, although the three genotype groups did not differ significantly in tHcy concentration at baseline, subjects with the TT genotype still responded with a larger decrease in tHcy levels than did those without the mutation. The present study, which included a total of 16 644 participants and is by far the largest study of its kind, concluded that even with the adjustment of baseline tHcy, folate and other important covariates, those participants with the TT genotype had a stronger tHcy reduction than those with either the CC or TT genotypes. However, the modifying effect of the MTHFR C677T genotype was weaker and became insignificant in participants with lower baseline tHcy levels. Furthermore, a lower degree of tHcy increase in participants with lower baseline tHcy (Fig. 3) and a greater degree of tHcy reduction in participants with higher baseline tHcy (Fig. 2) was also found in those with lower folate levels, eGFR <60 ml/min per 1·73 m2, males and ever smokers, independent of baseline tHcy levels. However, more studies are needed to confirm our results, and to further detect the underlying mechanisms involved in these modifying effects.
In folate-replete populations, the main determinant of elevated tHcy is metabolic vitamin B12 deficiency( Reference Robertson, Iemolo and Stabler 16 , Reference Quinlivan, McPartlin and McNulty 26 ). This is very common and quite often missed because physicians tend to assume incorrectly that a serum B12 in the reference range represents adequacy of B12 function( Reference Spence, Yi and Hankey 27 ). In the present study, the tHcy-lowering effect of folic acid therapy was very attenuated and became insignificant in those with metabolic B12 deficiency. These results highlight the importance of using vitamin B12 in addition to folic acid to lower tHcy. Moreover, methylcobalamin should be used in place of cyanocobalamin( Reference Spence, Urquhart and Bang 28 ), particularly in persons with impaired renal function (which includes the elderly)( Reference Qin, Zhang and Cai 29 ).
The mean tHcy reduction (−1·6 μmol/l) in the CSPPT is relatively small compared with other published trials; we offer the following explanations. First, the CSPPT supplemented with folic acid alone, whereas most other studies combined folate, B6 and/or B12 ( Reference Huo, Qin and Wang 10 – Reference Zhao, Wu and Li 12 ). The addition of other B vitamins may have helped to further lower the tHcy levels in these studies (about 7 % more than folic acid supplementation alone)( 13 ). Second, there are important differences in characteristics between the participants of the CSPPT and those of previously published trials, including younger age, lower percentage of males and relatively higher eGFR levels( Reference Huo, Qin and Wang 10 – Reference Zhao, Wu and Li 12 ). We have found that these factors can significantly modify the effect of folic acid supplementation in lowering tHcy. Last, in the CSPPT, there was a 54 % increase in folate level in the control group during the course of the trial( Reference Huo, Li and Qin 14 ). The cause of this increase is unclear. Over the course of the study, subjects received nutritional health education, which may have led to improved dietary choices( Reference Qin, Zhang and Cai 29 , Reference Qin, Li and Spence 30 ). Regardless of the cause, this change diminished the contrast between the two treatment groups and probably attenuated the effect estimation. Therefore, the potential true effect of folic acid supplementation on tHcy reduction could be underestimated in this setting.
The CSPPT found that the combined use of enalapril and folic acid, compared with enalapril alone, significantly reduced the risk of first stroke by 21 % (hazard ratio (HR) 0·79; 95 % CI 0·68, 0·93) among hypertensive patients( Reference Huo, Li and Qin 14 ). Our previous post hoc analysis of the CSPPT demonstrated that percent decline in tHcy was significantly associated with a reduction in stroke risk in hypertensive patients( Reference Zhao, Wu and Li 12 ). When percentage decline in tHcy was assessed as tertiles, a significantly lower stroke risk was found in those in tertiles 2 and 3 (HR 0·79; 95 % CI 0·64, 0·97) compared with participants in tertile 1( Reference Zhao, Wu and Li 12 ). Accordingly, tHcy reduction can be considered a reliable biomarker that indicates the beneficial effect of folic acid therapy in the prevention of stroke. However, in the CSPPT, the group difference in tHcy was only 1·6 μmol/l (about an 11 % reduction); therefore, we speculate that tHcy reduction may only partially explain the stroke risk reduction associated with folic acid therapy. We cannot ignore the direct antioxidant and antithrombotic effects of folate( Reference Verhaar, Stroes and Rabelink 31 ). More studies and analyses are needed to further illuminate the benefits of folate, independent of tHcy lowering, on the risk of stroke.
Our study had several strengths. First, the CSPPT included hypertensive participants without pre-existing stroke or MI. The low vascular disease burden and the low frequency of cardiac and vascular protective drug use made our results less likely to be confounded by these drugs. Second, the CSPPT, which had a large sample size and obtained individual data on MTHFR C677T genotypes, folate, vitamin B12, tHcy levels and other important factors at baseline, offers an exceptional opportunity to comprehensively evaluate potential effect modifiers for the tHcy-lowering effect of folic acid supplementation. Third, the CSPPT measured tHcy levels for 98·7 % of participants at baseline and 81·5 % of participants at the exit visit( Reference Huo, Li and Qin 14 ). Most relevant trials, however, only measured tHcy in a fraction of the total participants( Reference Albert, Cook and Gaziano 32 – Reference Bostom, Carpenter and Kusek 34 ). Therefore, the tHcy levels reported by these trials might not represent the whole spectrum of folate and tHcy levels in the total participants.
The present study also had some limitations. First, this study focused on adults with hypertension; the generalisability of our results to adults without hypertension remains to be determined. However, SBP both at baseline and over the treatment period did not significantly affect the tHcy-lowering effect of folic acid supplementation. Second, we did not measure other polymorphisms in the folate pathway, such as the MTHFR A1298C variant. Overall, our results still need to be investigated and confirmed in future studies.
In conclusion, long-term daily 0·8mg folic acid supplementation can significantly lower tHcy levels in a population of Chinese hypertensive patients with no history of CVD. More importantly, the degree of tHcy reduction is highly variable, and can be significantly affected by sex, MTHFR C677T genotypes, baseline folate, eGFR and smoking status. All these factors should be taken into account in the identification of participants who may be most likely to benefit from therapy to lower tHcy. The present study, therefore, provides useful data to inform the debate on folic acid fortification for public health reasons and folic acid supplementation for the prevention of a variety of clinical outcomes.
Acknowledgements
This work was supported by the National Key Research and Development Program (2016YFC0903103, 2016YFC0904900, 2016YFE0205400); the Science and Technology Planning Project of Guangzhou, China (201707020010); and the Science, Technology and Innovation Committee of Shenzhen (KQCX20120816105958775, JSGG20160229173428526, JSGG20170412155639040, GJHS20170314114526143, KC2014JSCX0071A); President Foundation of Nanfang Hospital, Southern Medical University (2017C007); Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2017J009).
The funders had no role in the design and conduct of the study (data collection, management, analysis and interpretation); the preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
X. Q., X. X., B. W., Y. L., X. H., J. L., Y. Z., Y. C., M. H., G. T., D. Y., J. L. and Y. H. contributed to research idea and study design. B. W., Y. L., X. H., H. W., Q. B., L. C., J. L., Y. Z., M. H., G. T., D. Y., Y. H. and X. Q. were involved in data acquisition. B. W., Y. L., X. H., H. W., Q. B., L. C. and X. Q. were involved in data analysis and interpretation. B. W., H. W., Y. L. and X. Q. performed statistical analysis. B. W., H. W., Y. L., X. H., Q. B., L. C., J. L., Y. Z., Y. C., M. H., G. T., D. Y., J. L., Y. H., X. Q. and X. X. carried out review and revision of the article.
X. X. reports grants from the Projects of the National Natural Science Foundation of China; the Science and Technology Planning Project of Guangzhou, China; and the Science, Technology and Innovation Committee of Shenzhen. B. W. reports grants from the National Key Research and Development Program. Y. H. reports grants from the National Key Research and Development Program. Y. C. reports grants from National Key Technologies Research and Development Program. X. Q. reports grants from Nanfang Hospital, Southern Medical University. Y. L. reports grants from Nanfang Hospital, Southern Medical University. No other disclosures were reported.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518002477










