Tannins are polyphenolic compounds and are widespread in the plant kingdom(Reference Yoshida, Hatano and Ito1). They can be classified into two groups: hydrolysable tannins, which contain gallic acids and their oxidation products(Reference Mueller-Harvey2, Reference Hartzfeld, Forkner and Hunter3), and condensed tannins, which are oligomers and polymers of flavan-3-ols(Reference Porter, Hemingway and Laks4). Tannins possess diverse biological effects because they are metal ion chelators(Reference McDonald, Mila and Scalbert5), protein precipitating agents(Reference Santos-Buelga and Scalbert6) and antioxidants(Reference Hagerman, Riedl and Jones7).
Recent studies have shown that tannins generate antioxidant effects in vivo in different animal tissues. Luciano et al. (Reference Luciano, Vasta and Monahan8) have reported an improvement in the antioxidant status of muscle from lambs fed a concentrate-based diet supplemented with tannins from a quebracho (Schinopsis lorentzii) extract compared with a control tannin-free diet. In another study, Luciano et al. (Reference Luciano, Monahan and Vasta9) reported that quebracho tannins in lamb diets can improve meat colour stability by delaying myoglobin oxidation during refrigerated storage. In these studies, however, the authors did not assess whether the antioxidant effect of dietary tannins was direct, due to a transfer of phenolic compounds from feed to muscle.
It is known that many hydrolysable tannins can be degraded by micro-organisms in the rumen(Reference McSweeny, Palmer and McNeill10–Reference Goel, Puniya and Aguilar15). However, it is unclear to what extent microbial organisms degrade or metabolise condensed tannins. Several publications have reported that condensed tannins cannot be degraded(Reference Makkar, Becker and Abel16–Reference Bhat, Makkar and Singh18) by ruminal micro-organisms. In contrast, Perez-Maldonado & Norton(Reference Perez-Maldonado and Norton19) suggested that condensed tannins could be absorbed or degraded during metabolism in the gastrointestinal tract in sheep and goats. Furthermore, it is unclear whether these findings apply to all tannins or whether condensed tannins differ in their susceptibilities to microbial degradation.
The aim of the present study was to investigate whether quebracho tannins or their metabolites could be detected in the tissues of lambs fed a diet supplemented with quebracho extract. To achieve this objective, liver and plasma samples underwent a solid-phase extraction (SPE) step in order to purify and concentrate phenolic compounds from the samples, which were then analysed by liquid chromatography (LC)–MS. The antioxidant capacity of the samples purified with SPE was measured in order to assess whether the possible presence of phenolic compounds in tissues could affect their antioxidant status. Furthermore, the antioxidant status was measured in samples that had not been purified by SPE. These experiments sought to investigate whether the antioxidant effects of dietary quebracho tannins in lambs, which had been previously reported(Reference Luciano, Vasta and Monahan8, Reference Luciano, Monahan and Vasta9), could be related to their transfer to animal tissues.
Materials and methods
The experimental protocol of the study, including all procedures involving animals, was approved by the University of Catania under the project Ricerca di Ateneo 2008 and by the Italian Ministry of University and Research under the project PRIN 2007 (protocol 200778K3KJ_003).
Animals and diets
A total of eighteen Comisana lambs were weaned at 45 d of age (mean weight 14·48 (sd 2·41) kg). Lambs were blocked in groups of two on a descending body weight basis and, within block, were assigned to one of the two dietary treatments of nine animals each (C, control and C+T, control+tannins) and kept in individual pens for the duration of the trial (randomised complete block design). The C group received a concentrate containing the following ingredients: barley (55·1 %), alfalfa hay (30·0 %), soyabean meal (13·0 %) and vitamin and mineral premix (1·9 %). The C+T group received the concentrate plus supplementary quebracho tannins (from S. lorentzii; Figli di Guido Lapi S.pA.). Each 1000 g of DM of concentrate plus tannins consisted of 95·7 g quebracho powder and 904·3 g of concentrate. The quebracho-supplemented diet was formulated to contain 6·4 % (DM basis) tannins. The lambs were adapted to the experimental diets for 7 d before the commencement of the experiment. After 70 d of experiment, the lambs were slaughtered. All lambs had access to the diets until 1 h before slaughtering.
Sampling
On the day before slaughtering, individual blood samples were taken from the jugular vein of each lamb after 12 h of fasting and collected in heparin tubes. Blood samples were centrifuged at 3000 g for 20 min at 4°C and stored at − 80°C. The livers, taken after slaughtering, were immediately frozen in liquid N2, vacuum packed and stored at − 20°C. Each type of feed was prepared in a unique batch at the beginning of the trial. Sub-samples were taken fortnightly, vacuum packed and frozen at − 20°C. A uniform and representative sample of each diet was finally prepared for the analysis.
Extraction, purification and identification of phenolic compounds
Phenolic compounds were purified and isolated from feed and animal tissues by SPE and then identified by LC–MS analysis (see later).
Preparation of feed samples and purification of phenolic compounds by solid-phase extraction
Feeds (2·5 g) given to both C and C+T lamb groups were placed into 50 ml centrifuge tubes. Samples were homogenised with 15 ml acetone–water (70–30, v/v) for 60 s at 4000 rpm using a Heidolph Diax 900 tissue homogeniser (Heidolph Elektro GmbH & Company KG). Samples were then sonicated for 6 min (with a break of 2 min after the first 3 min of sonication) using a Bandelin Sonoplus HD2070 sonicator (cycle: 4 × 10 %, power: 0·31 %). Samples were kept in a water/ice bath during both homogenisation and sonication procedures. The sonicated homogenates were centrifuged at 3000 g for 15 min at 4°C using a centrifuge (model IEC CL31R; Thermo Fisher Scientific). Then, the supernatants were filtered through Whatman 541 filter paper before SPE purification.
Phenolic compounds were isolated from feed samples on a C18 Sep-Pak Vac 6cc (500 mg) cartridge (WAT043395; WATERS SpA). The method was based on that described by Pérez-Magariño et al. (Reference Pérez-Magariño, Ortega-Heras and Cano-Mozo20) but was adapted as follows. Prior to use, cartridges were conditioned with 3 ml methanol followed by 3 ml of distilled water. The filtered supernatant (10 ml) was acidified to pH 2·5 with 0·5 m-H2SO4 prior to loading onto the cartridge. Phenolic compounds were eluted with 3 ml of methanol and the final fraction was divided into two 1·5 ml glass vials and kept in a freezer at − 30°C.
Preparation of lamb tissues and purification of phenolic compounds by solid-phase extraction
Liver (5 g) was placed into a 50 ml centrifuge tube. Preparation steps for the liver samples were carried out in the same way as for the feed samples. The SPE method was based on that described by Pérez-Magariño et al. (Reference Pérez-Magariño, Ortega-Heras and Cano-Mozo20) and carried out for feed samples. For liver samples, phenolic compounds were eluted with 3 ml of ethyl acetate. The collected fraction was evaporated to dryness under N2 and then dissolved in 3 ml of methanol. The final fraction was also divided into two 1·5 ml glass vials and kept at − 30°C.
The method for the plasma samples was based on that described by Juan et al. (Reference Juan, Maijó and Planas21) but was adapted as follows. A 500 μl plasma aliquot was acidified with 15 μl of glacial acetic acid. Phenolic compounds were isolated from plasma samples on a C18 Sep-Pak 1cc (100 mg) cartridge (WAT023590; WATERS SpA). Prior to use, the cartridges were conditioned with 3 ml of methanol followed with 3 ml of distilled water. The acidified plasma sample was completely loaded onto the cartridge followed by 1 ml of distilled water. Phenolic compounds were eluted with 2 ml of methanol. Aqueous ascorbic acid (10 μl; 15 %, w/v) was added to the final eluate to prevent oxidation in plasma samples. This fraction was evaporated to dryness under N2 and then dissolved in 3 ml of methanol. The final fraction was divided into two 1·5 ml sub-samples and stored at − 30°C. Samples were acidified in order to disrupt polyphenol–protein binding, as explained in Juan et al. (Reference Juan, Maijó and Planas21).
To confirm that the SPE method of Pérez-Magariño et al. (Reference Pérez-Magariño, Ortega-Heras and Cano-Mozo20) retained phenolic compounds, we tested the method in our laboratory as follows. Three solutions of known concentrations of gallic acid were tested in duplicate. Standard solutions with and without SPE treatment were analysed by the Folin–Ciocalteu assay, as described later for the plasma samples. Standard solutions also underwent the SPE treatment, performed as explained earlier for plasma samples, but without the addition of ascorbic acid to the final eluate. The eluate obtained after the SPE step was evaporated to dryness under N2 and then dissolved in 1·5 ml of methanol–distilled water (1:1, v/v). A volume of 500 μl of the final eluate was placed into a 15 ml centrifuge tube and 500 μl of methanol–distilled water (1:1, v/v) was added. A volume of 500 μl of standard solution without the SPE treatment was also placed into a 15 ml centrifuge tube and 500 μl of methanol–distilled water (1:1, v/v) was added. In all standard solutions (with and without SPE treatment), Folin–Ciocalteu reagent was diluted to 1 N and 500 μl were added to the tubes followed by 2·5 ml aqueous solution of sodium carbonate (20 %, w/v). The Folin–Ciocalteu assay was performed as described later for Folin–Ciocalteu assay in RAW samples. The results showed an 86·35 % recovery of gallic acid with a ± 7·47 of variability.
Feed and tissue extracts liquid chromatography–MS analysis
Feed and lamb tissue extracts were analysed by HPLC–MS using an ACE 5 2·1 × 150 mm C18 column (Hichrom Limited) fitted to an Agilent 1100 liquid chromatograph with a diode array detector. A binary mobile phase system was used where solvent A was HPLC-grade water +0·1 % formic acid and solvent B was HPLC S-grade acetonitrile+0·1 % formic acid (Rathburn Chemicals Limited). Metabolites were eluted from the column using a simple gradient programme. Initial conditions were 95 % A and 5 % B held for 1 min, changing to 5 % A and 95 % B over 9 min and then held for 5 min before returning to the initial gradient conditions over 1 min and then held for 9 min to re-equilibrate the column. The pump flow rate was 0·2 ml/min and the column oven temperature was 25°C.
For LC–MS analysis, 5 μl of each sample were injected and the eluted peaks were analysed using an electrospray ionisation microTOF QII quadrupole time of flight (TOF) mass spectrometer (Bruker Daltonics) operated in the negative ion mode using a capillary voltage of 3200 V, nebuliser gas (N2) pressure of 1 bar, dry gas (N2) flow of 8 l/min and a drying temperature of 180°C. The TOF tube was set at +8600 V and the detector at 2010 V. The mass range, of 100–1700 Da, was calibrated using Agilent low concentration Tunemix (G1969–85 000). A volume of 5 μl of a standard catechin solution (10 ng/μl) was first injected followed by a blank with each batch of samples to check system integrity and performance. Peak areas of lipophilic compounds were normalised based on catechin areas. The method was based on that described by Wright et al. (Reference Wright, Gibson and Spencer22) but was adapted as just mentioned.
Antioxidant status of liver and plasma samples treated with or without solid-phase extraction
Liver and plasma antioxidant status was determined by means of the ferric-reducing antioxidant power (FRAP) and Folin–Ciocalteu assays. Both assays were applied to samples either treated (SPE samples) or not treated (RAW samples) with SPE. All samples were analysed in duplicate for Folin–Ciocalteu and FRAP assays.
Folin–Ciocalteu assay on RAW samples
For the preparation of RAW-liver for the Folin–Ciocalteu assay, 2 g of the liver was placed into a 50 ml centrifuge tube and homogenised with 10 ml of distilled water. Homogenisation, sonication, centrifugation and filtration steps were performed as described earlier for SPE liver. A 1:4 dilution of the extract (3 ml of distilled water added to 1 ml of liver extract) was chosen. The assay was performed as described by Luciano et al. (Reference Luciano, Vasta and Monahan8). Briefly, 100 μl of the diluted RAW-liver extract were transferred into 15 ml centrifuge tubes and 900 μl of distilled water was added. The Folin–Ciocalteu reagent was diluted to 1 N and 500 μl were added to the tubes followed by 2·5 ml aqueous solution of sodium carbonate (20 %, w/v). The mixture was vortex mixed for 30 s and incubated for 40 min in the dark at room temperature. The samples were centrifuged at 2700 g for 10 min at 4°C in order to remove any sodium carbonate precipitates. A double-beam spectrophotometer (model UV-1601; Shimadzu Corporation) was used to measure the absorbance of the samples. The wavelength used was 725 nm and a tube containing all the reagents except the tissue extract was used as a blank. Aqueous solutions of gallic acid were used for the calibration curve. The concentration range for the calibration curve covered 0 to 80 μg/μl of gallic acid. The results were expressed as mg of gallic acid equivalents/g of liver. For RAW-plasma samples, 100 μl of plasma diluted in the ratio 1:10 with distilled water were taken in 15 ml centrifuge tubes and 900 μl of distilled water were added. The Folin–Ciocalteu assay was carried out as described for RAW-liver samples. The results were expressed as mg of gallic acid equivalents/ml of plasma.
Folin–Ciocalteu assay of solid-phase extraction-treated samples
The liver and plasma samples treated with SPE were subjected to the Folin–Ciocalteu assay as follows. The content of one of the two 1·5 ml glass vials obtained after the SPE step was evaporated to dryness under N2 and then dissolved in 1·5 ml of methanol–distilled water (1:1, v/v). In the case of SPE-liver samples, 500 μl of this extract were transferred into a 15 ml centrifuge tube and 500 μl of methanol–distilled water (1:1, v/v) were added. The Folin–Ciocalteu assay was performed as described earlier for RAW-liver. Solutions of gallic acid in 1:1 (v/v) methanol–distilled water were used to calibrate the assay.
For measuring the total phenolic content and the antioxidant status in plasma, SPE-plasma samples were obtained as described earlier, with the only difference that ascorbic acid was not added to the final sample; in fact, Georgé et al. (Reference Georgé, Brat and Alter23) showed that ascorbic acid interferes in the Folin–Ciocalteu assay. The final SPE-plasma extracts were evaporated to dryness under N2 and then dissolved in 3 ml of methanol–distilled water (1:1, v/v); a volume of 500 μl of this sample was placed into a 15 ml centrifuge tube and 500 μl of methanol–distilled water (1:1, v/v) were added. The Folin–Ciocalteu assay was performed as described for RAW-liver. The results were expressed as mg of gallic acid equivalents/ml of plasma.
Ferric-reducing antioxidant power assay of RAW samples
The method described by Luciano et al. (Reference Luciano, Vasta and Monahan8) was followed to measure the FRAP. The FRAP reagent was prepared by mixing ten volumes of acetate buffer (300 mm, pH 3·6) with one volume of a solution of 2,4,6-tripyridyl-S-triazine (TPTZ; 10 mm in 40 mm-HCl) and with one volume of 20 mm-aqueous ferric chloride. A blank reading at 593 nm was taken immediately after mixing 400 μl of distilled water with 3 ml of FRAP reagent. For RAW-liver samples, 0·5 g of liver were placed into a 50 ml centrifuge tube and 10 ml of distilled water were added. Homogenisation, sonication, centrifugation and filtration steps were performed as described earlier for SPE-liver samples. Then, in a glass test tube, 300 μl of distilled water were mixed with 100 μl of liver extract and 3 ml of warm FRAP reagent (37°C) were added. The contents of the tube were mixed and incubated in a water bath set at 37°C for 4 min, after which the absorbance was recorded at 593 nm. The change in absorbance (ΔA 593nm) between the final reading and the blank reading was related to that obtained with solutions of Fe2+ of known concentrations (aqueous FeSO4.7H2O ranging from 0 to 1000 μm). Results of the FRAP assay were therefore expressed as μmol of Fe2+ equivalents/g of liver. For RAW-plasma samples, 74 μl of plasma were added in a glass test tube containing 220 μl of distilled water and 2·2 ml of FRAP reagent (the final dilution of the sample in the mixture was always 1:34). Tubes were mixed and incubated for 4 min in a water bath at 37°C. The absorbance was immediately recorded at 593 nm. Results of the FRAP assay were therefore expressed as μmol of Fe2+ equivalents/ml of plasma.
Ferric-reducing antioxidant power assay in solid-phase extraction samples
The same method described earlier was applied to the SPE samples. Since SPE samples were prepared in a methanol–distilled water solution, the same solution was used instead of distilled water in the assay, as well as for preparing standard FeSO4.7H2O solutions. The assay was performed by adding in a glass test tube 200 μl of the SPE-liver samples or SPE-plasma samples (without ascorbic acid) to 200 μl of methanol–distilled water and 3 ml of FRAP reagent. Incubation time, absorbance measurement and calculations were performed as described earlier.
Statistical analyses
The data for the lipophilic compounds were analysed by one-way ANOVA to test the effect of the dietary treatment (C v. C+T) and experimental error.
To test the effects of the SPE method used and of the dietary supplementation with tannin extract, the data of Folin–Ciocalteu and FRAP assays performed on the liver and plasma were analysed as repeated measures using the MIXED procedure of the statistical software SAS (version 9). The fixed effects tested in the model were the dietary treatment (Diet; C or C+T), the purification or not with SPE (RAW samples or SPE samples) and their interaction (Diet × SPE). The individual lamb was included in the model as a random effect nested with the Diet. The covariance structure used was the variance components. Multiple comparisons of the least squares means, presented in Table 3, were performed using the PDIFF option of SAS and the Tukey's adjustment for multiple comparisons was adopted.
In all the statistical analyses performed, significance was declared for P ≤0·05; when 0·05 < P ≤0·1, the means were considered as different in tendency.
Results
Analysis of feed samples by liquid chromatography–MS
Representative chromatograms of quebracho-enriched and control diets are shown in Fig. S1 of the supplementary material (available online). The quebracho chromatogram differed from the control by the presence of one peak at 12·1 min. The extracted ion chromatogram revealed that this peak contained several different masses (see Fig. S2 of the supplementary material, available online) with m/z (M − H)− of 561·150, 833·223 and 1105·293. These compounds were identified as a combination of one or more fisetinidol units plus one catechin unit (see Fig. S3 of the supplementary material (available online); Table 1). Another single peak was observed at 12·8 min with an m/z (M − H)− of 285·046 and was identified as fisetin (see Fig. S4 of the supplementary material (available online); Table 1). LC–MS analysis of the control diet revealed the absence of the compounds identified in the quebracho-enriched diet.
Table 1 Main phenolic compounds identified in the quebracho-enriched diet by liquid chromatography–MS
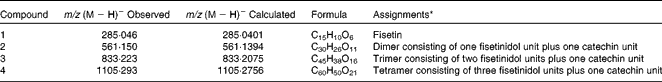
* See Fig. S3 of the supplementary material (available online) for chemical structures.
Analysis of animal tissues by liquid chromatography–MS
Plasma and the liver extracts obtained after the SPE step were analysed for quebracho profisetinidins and other phenolic compounds or possible metabolites arising from their degradation. Under the LC–MS conditions used in the present study, phenolic compounds were expected to elute between 5 and 12 min. However, no signals from any of these compounds could be detected in the liver (see Fig. S5 of the supplementary material, available online) or plasma (see Fig. S6 of the supplementary material, available online) samples from lambs fed the C+T diet. Liver chromatograms from lambs fed the C or C+T diet were exactly the same. In plasma chromatograms, no differences could be found between the lambs fed with the different diets. In plasma chromatograms, the peak at 2·5 min is ascorbic acid, which had been added during the SPE step. Although in plasma chromatograms (Fig. S6, available online) some peaks were observed between 5 and 12 min, these peaks belonged to nitrogen-containing compounds rather than phenolic compounds. For this reason, these compounds were not analysed further. The peaks observed between 12 and 25 min in the chromatograms from the plasma and the liver samples are due to lipophilic compounds (Table 2); the plasma of the C+T lambs presented greater (P ≤0·008) amounts of C18H30O2, C18H32O2, and C20H30O2 and the compound C18H34O2 showed a tendency (P =0·09) compared with the C animals. No significant differences (P>0·05) were found in the amounts of the lipophilic compounds detected in the liver samples of the lambs from the two groups.
Table 2 Lipophilic compounds* found in the liver and plasma of lambs fed concentrate (C) or C plus quebracho tannins (C+T)
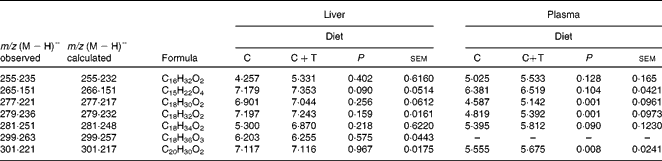
* Data expressed as log of peak area.
Folin–Ciocalteu assay in lamb tissues
As shown in Table 3, the SPE purification procedure (P< 0·0001) had an effect on the results from the Folin–Ciocalteu assay, which were obtained from the liver samples. Lower values were measured in the SPE samples compared with the RAW samples, regardless of the dietary treatment. The dietary treatment tended to affect the values of the Folin–Ciocalteu assay of the liver samples (P =0·062); however, tendency was detectable in the Diet × SPE interaction (P =0·061). The multiple comparisons test showed that the RAW samples from animals fed the C+T diet tended to have higher values of the Folin–Ciocalteu assay compared with those from the lambs fed the C diet (+9·93 %, P =0·073; Table 3). Conversely, no difference in the Folin–Ciocalteu values was observed between the C and C+T groups when the liver samples underwent the SPE purification step (SPE samples). A similar trend was observed more clearly when the Folin–Ciocalteu assay was performed on the plasma samples, with values of the SPE samples being significantly lower compared with those measured in the RAW samples (P< 0·0001). A significant effect of Diet was found, as well as a significant Diet × SPE interaction (P =0·005 and 0·008, respectively; Table 3). Multiple comparisons between means showed that RAW-plasma samples from lambs fed the C+T diet had higher Folin–Ciocalteu values as compared with samples from lambs fed the C diet (+5·86 %, P =0·003; Table 3). However, no difference between the C- and the C+T-fed animals was found when the plasma was subjected to the SPE treatment.
Table 3 Folin–Ciocalteu and ferric-reducing antioxidant power (FRAP) values measured on RAW samples and solid phase extraction (SPE) samples (Least square mean values with their pooled standard errors)
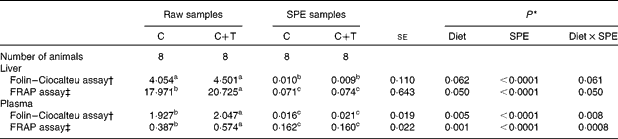
C, concentrate; C+T, C plus quebracho tannins.
a,b,cMean values within a row with unlike superscript letters were significantly different between least squares means (P< 0·05) tested using the Tukey's adjustment for multiple comparisons.
* P values of the test of the fixed effects performed with the mixed procedure statistical analysis. The fixed effects tested were dietary treatment (Diet; C or C+T), SPE treatment (SPE; RAW samples or SPE samples) and their interaction (Diet × SPE).
† Expressed as mg of gallic acid equivalents (GAE)/g of liver, or as mg of GAE/ml of plasma.
‡ Expressed as μmol of Fe2+ equivalents/g of liver, or as μmol of Fe2+ equivalents/ml of plasma.
Ferric-reducing antioxidant power assay in lamb tissues
As shown in Table 3, the SPE purification procedure strongly reduced the values of the FRAP assay in the liver samples, regardless of the dietary treatment (P< 0·0001). Diet significantly affected the FRAP values of the liver (P =0·050); however, a significant Diet × SPE interaction (P =0·050) showed how the Diet effect was dependent on the purification of the samples by the SPE. Indeed, multiple comparisons showed that FRAP values were higher in RAW-liver samples from lambs fed the C+T diet compared with the C diet (+13·28 %, P =0·050; Table 3); conversely, no difference between the C+T and C diets were observed in the SPE-liver samples. Similarly, the SPE purification overall reduced the FRAP values measured in plasma (P< 0·0001), an effect of Diet was found (P =0·001), as well as a Diet × SPE interaction (P =0·0008). As observed in the liver samples, the C+T diet resulted in higher FRAP values in the RAW-plasma samples compared with the C diet (+32·57 %, P =0·0002; Table 3). However, there was no difference in FRAP values between the C and C+T diets in the SPE-plasma samples.
Discussion
To the best of our knowledge, the present study is the first aiming to determine the metabolic fate of quebracho tannins after being ingested by ruminants. So far, the use of quebracho in ruminant diets has been investigated with respect to their toxicity(Reference Hervás, Pérez and Giráldez24), their anthelmintic effect(Reference Athanasiadou, Kyriazakis and Jackson25), N utilisation(Reference Waghorn, Shelton and McNabb26), their role in lipid metabolism(Reference Vasta, Mele and Scerra27) and meat colour(Reference Luciano, Vasta and Monahan8, Reference Luciano, Monahan and Vasta9). Vasta et al. (Reference Vasta, Priolo and Scerra28) reported that supplementing lambs with quebracho tannins increased the expression in the longissimus dorsi muscle of the Δ9 desaturase enzyme compared with lambs fed the same diet but without a quebracho supplement. Luciano et al. (Reference Luciano, Vasta and Monahan8, Reference Luciano, Monahan and Vasta9) reported that supplementary quebracho increased muscle antioxidant status and reduced the discolouration in lamb meat stored for up to 11 d in a modified atmosphere(Reference Luciano, Monahan and Vasta9) or up to 7 d in aerobic conditions(Reference Luciano, Vasta and Monahan8). These effects observed in muscles imply that dietary quebracho tannins can affect the post-digestive metabolism. Two possible mechanisms could explain these observations: (i) ingested quebracho tannins (or their metabolites) might be degraded and absorbed from the ruminant digestive tract before being transferred to tissues or (ii) dietary quebracho tannins (and their metabolites) are not absorbed in the digestive tract but act instead as antioxidants in the gastrointestinal tract(Reference Kerem, Chertrit and Shoseyov29). It is well-known that tannins form complexes with proteins and interfere with ruminal digestion in general. Protein complexation affects but does not eliminate the antioxidant activities of tannins(Reference Riedl and Hagerman30, Reference Arts, Haenen and Wilms31). Therefore, it is possible that tannins may indirectly affect muscle biochemistry via some other components. In the light of these speculations, the present study investigated whether or not dietary quebracho tannins or their metabolites were present in lamb plasma and liver.
The main types of tannins found in the quebracho-enriched diet were profisetinidins (Table 1) and have been described previously in quebracho extracts(Reference Roux, Hemingway and Laks32, Reference Mueller-Harvey33). It should be noted that, in contrast to most other condensed tannins, profisetinidins do not contain 5-OH groups in close proximity to the interflavanol bond. The absence of this 5-OH group increases the stability of the interflavanol linkages in condensed tannins(Reference Mueller-Harvey, Caygill and Mueller-Harvey34), and therefore quebracho tannins are particularly difficult to degrade.
The bioavailability of phenolic compounds in ruminants has been only marginally investigated. Gladine et al. (Reference Gladine, Rock and Morand35) found monomeric phenol compounds in plasma when the sheep had received polyphenol-rich plant (i.e. grape or rosemary) extracts by ruminal infusion. Moñino et al. (Reference Moñino, Martínez and Sotomayor36) reported that the muscle of lambs receiving the milk of ewes fed with a rosemary-enriched concentrate contained several of the phenols that were present in the diet of the ewe. These studies suggested that only some phenolic compounds are bioavailable. Clearly, the chemical structures of tannins and phenolic acids studied by Gladine et al. (Reference Gladine, Rock and Morand35) and Moñino et al. (Reference Moñino, Martínez and Sotomayor36) differ from the profisitenidins investigated in the present study (see Fig. S3 of the supplementary material, available online)
The present study demonstrated that, with the analytical procedures adopted, no phenolic compounds could be detected in lamb tissues, which leads us to consider that the profisetinidin tannins from quebracho might not be degraded or absorbed in the gastrointestinal tract. This agrees with Makkar et al. (Reference Makkar, Becker and Abel16) who reported that quebracho tannins, as measured by the butanol–HCl–Fe3+ reagent(Reference Porter, Hrstich and Chan37), are not degraded by ruminal micro-organisms in an in vitro study. Therefore, it is likely that these types of tannins are directly eliminated through the faeces. Nevertheless, other authors reported that ruminal microflora were able to degrade quebracho tannins into smaller phenolic compounds(Reference Bhat, Singh and Sharma38).
We found that the RAW-liver and RAW-plasma samples of the C+T lambs displayed higher FRAP and Folin–Ciocalteu values than the samples from the control lambs. These results agree with those reported by Luciano et al. (Reference Luciano, Vasta and Monahan8) who showed that tannins from quebracho increased the antioxidant status in lamb muscle measured as Folin–Ciocalteu or FRAP values. However, the Folin–Ciocalteu and the FRAP reagents are not specific to phenolic compounds and react to a wide spectrum of reducing compounds(Reference Wright, Gibson and Spencer22), while the SPE method used is highly selective for the isolation and concentration of phenolic compounds(Reference Pérez-Magariño, Ortega-Heras and Cano-Mozo20). The much lower FRAP and Folin–Ciocalteu values found here in the SPE samples compared with RAW samples are likely to be due to the removal of antioxidants other than phenolic compounds by the SPE treatment. Moreover, when the liver and the plasma extracts were passed through SPE cartridges, no difference in the Folin–Ciocalteu and FRAP tests was found between lambs fed with the control diet or with the quebracho-enriched diet. In the light of this, the higher antioxidant capacity of RAW-liver and RAW-plasma from lambs fed the C+T diet suggests that dietary tannins are able to positively affect the antioxidant status of the animal tissues. However, the lack of difference between the C and C+T treatments in the antioxidant capacity measured after SPE treatment of the liver and the plasma samples, together with the absence of phenolic compounds in these tissues, leads us to believe that the antioxidant effects of dietary quebracho tannins were not directly related to their absorption and deposition in the tissues. Rather, the improved antioxidant capacity of the liver and the plasma from lambs fed quebracho appears to result from unknown and indirect antioxidant mechanisms(Reference Vasta and Luciano39). These effects could be mediated, for example, by a direct antioxidant activity of the tannins in the gastrointestinal tract, such as a removal or chelation of pro-oxidant compounds and a reduction of lipid peroxidation, which would result in an overall improvement of the animal's antioxidant status(Reference Kerem, Chertrit and Shoseyov29, Reference Halliwell, Rafter and Jenner40). Furthermore, it is known that dietary condensed tannins strongly modify lipid metabolism in ruminants(Reference Vasta, Mele and Scerra27) and also interfere with gene(Reference Kresty, Howell and Maureen41) and protein expression(Reference Vasta, Priolo and Scerra28). In particular, Sgorlon et al. (Reference Sgorlon, Stradaioli and Zanin42) found that supplementing sheep with grape skin extract, which is rich in polyphenols and condensed tannins, increased the expression in plasma of the superoxide dismutase enzyme, which is involved in the endogenous antioxidant defence system. Interestingly, Larrosa et al. (Reference Larrosa, García-Conesa and Espín43) recently reported that hydrolysable tannins also resulted in indirect antioxidant mechanisms in monogastric animals.
The lipophilic compounds detected in the liver and the plasma are likely to be fatty acids and, in particular, the formulae C18H30O2, C18H32O2 and C20H30O2 could correspond to C18 and C20 PUFA and C18H34O2 could correspond to a MUFA. The plasma of the C+T lambs contained more of these compounds than the plasma of C lambs (Table 2). It is known that feeding tannins increases PUFA accumulation in the tissues(Reference Vasta, Mele and Scerra27); this is due to a reduced biohydrogenation of PUFA in the rumen, as tannins depress the relative abundance of some bacterial strains responsible for the biohydrogenation(Reference Athanasiadou, Kyriazakis and Jackson25).
In conclusion, with the analytical procedures adopted in the present study, no tannins or other phenolic compounds could be detected in the liver or the plasma samples when lambs were fed a quebracho-supplemented diet. This is in contrast with studies in which plant extracts rich in other types of polyphenols and tannins were given to lambs. The present results are likely to be due both to the very low amount of fisetin (a low-molecular-weight phenol) and to the structural stability of profisetinidins in quebracho. Although no phenolic compounds were detected in the liver and plasma of quebracho-fed lambs, these tissues showed a greater antioxidant capacity compared with the liver and plasma of unsupplemented lambs. Therefore, it can be concluded that supplementing quebracho tannins improves the antioxidant capacity of tissues via an indirect effect, possibly by enhancing the tissue endogenous antioxidant system or by participating in the regeneration of other antioxidant compounds.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114512005703
Acknowledgements
This research was funded by the University of Catania, Progetto di Ricerca di Ateneo, 2008 (main investigator L. B.) and by the Italian Ministry of Research, PRIN 2007, protocol 200778K3KJ_03, entitled Effects of Tannins on Sheep and Goat Meat Quality (main investigator, A. P.). P. L.-A. planned and performed all the analyses and wrote the manuscript. G. L. was responsible for the organisation of the in vivo experiment, assisted in the analyses of the antioxidant status parameters and in the analysis and discussion of the results. T. M. G. provided assistance for LC–MS analyses. I. M.-H. supervised all the activities at the University of Reading related to the optimisation of the analyses of phenolic compounds and contributed to the interpretation of the related results. V. V. supervised the optimisation of the SPE purification of phenolic compounds. The experiment was designed by A. P. and L. B. The authors gratefully acknowledge Dr Corrado Dimauro (Dipartimento di Scienze Zootecniche, University of Sassary, Italy) for assistance in data analyses. The authors declare no conflict of interest.







