Fish are an important source of n-3 long-chain PUFA (LC-PUFA) – namely, of EPA (20 : 5n-3) and DHA (22 : 6n-3) – which are considered of relevance to the health of human consumers. The n-3 LC-PUFA content of farmed fish has been guaranteed by the high incorporation levels of fish oil (FO) and fishmeal (FM) in aquafeeds, which are non-sustainable feed resources( Reference Bell, Henderson and Tocher 1 , 2 ). Thus, both from economic and environmental perspectives, a sustainable growth of the aquaculture sector demands that alternatives to these marine fishery-derived feedstuffs be used in aquafeeds. Plant feedstuffs (PF) and vegetable oils (VO) are among the most suitable potential alternatives to FM and FO. However, VO are devoid of n-3 LC-PUFA, which are characteristics of FO. In addition, increasing of VO levels in aquafeeds usually reduces dietary cholesterol content and increases phytosterol levels( Reference Bell, Henderson and Tocher 1 , Reference Liland, Espe and Rosenlund 3 ).
Over the years, a large body of information has been obtained concerning the effects of such ingredients on growth performance, feed efficiency, metabolic responses, health and nutritional quality of several fish species( Reference Leaver, Villeneuve and Obach 4 – Reference Morais, Edvardsen and Tocher 7 ). Data obtained so far indicate that FO in aquafeeds can be substantially replaced with VO without any major effects on fish performance( Reference Morais, Edvardsen and Tocher 7 – Reference Benedito-Palos, Navarro and Sitja-Bobadilla 11 ). However, VO are reported to affect fish body composition and lipid metabolism( Reference Leaver, Villeneuve and Obach 4 , Reference Geay, Ferraresso and Zambonino-Infante 6 , Reference Morais, Edvardsen and Tocher 7 ). The effects of VO on lipogenesis are not consistent( Reference Richard, Kaushik and Larroquet 9 , Reference Richard, Mourente and Kaushik 10 , Reference Regost, Arzel and Robin 12 – Reference Panserat, Kolditz and Richard 16 ), but obvious changes in other lipid metabolic processes such as LC-PUFA synthesis and cholesterol metabolism were reported( Reference Leaver, Villeneuve and Obach 4 , Reference Panserat, Kolditz and Richard 16 ).
Although salmonids and freshwater species are capable of bioconversion of C18 fatty acids (FA) to n-3 LC-PUFA, this endogenous synthesis seems to be inefficient to counteract the dietary n-3 LC-PUFA deficit in fish fed VO-based diets, as reduction of n-3 LC-PUFA levels in the flesh and a decrease in the final product nutritional quality are usually observed( Reference Bell, Henderson and Tocher 1 ). Nonetheless, in freshwater fish and salmonids fed with VO a clear up-regulation of desaturase gene expression and increased activity of the enzymes involved in the conversion of C18 FA to n-3 LC-PUFA are observed( Reference Bell, Henderson and Tocher 1 , Reference Seiliez, Panserat and Kaushik 17 , Reference Zheng, Tocher and Dickson 18 ). Such responses are not so obvious in marine fish( Reference Morais, Edvardsen and Tocher 7 , Reference Tocher, Zheng and Schlechtriem 19 , Reference González-Rovira, Mourente and Zheng 20 ).
Replacement of FM by PF will increase dietary carbohydrate content, as carbohydrates are present in high quantities in most PF. Carbohydrates are, however, almost absent in the natural food of most fish species and are not well utilised by fish, particularly by carnivorous species. Previous studies suggest that carbohydrates have an important role in the modulation of lipid metabolism in fish( Reference Seiliez, Panserat and Kaushik 17 , Reference Dias, Alvarez and Diez 21 – Reference Kamalam, Médale and Larroquet 25 ). For instance, carbohydrate administration alters plasma TAG( Reference Peres, Goncalves and Oliva-Teles 22 , Reference Enes, Peres and Sanchez-Gurmaches 23 ), induces hepatic lipogenesis and lipid deposition( Reference Dias, Alvarez and Diez 21 , Reference Kamalam, Medale and Kaushik 24 ), and stimulates FA bioconversion in salmonids( Reference Seiliez, Panserat and Kaushik 17 , Reference Kamalam, Médale and Larroquet 25 ). The latter aspect is of particular interest, as expression or increased activity of elongation and desaturation enzymes may contribute to increasing the amounts of n-3 LC-PUFA in fish tissues and hence the nutritional quality of fillets. Conversely, regulation of carbohydrate metabolism by dietary lipids was also reported in rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar L.)( Reference Panserat, Perrin and Kaushik 26 , Reference Menoyo, Diez and Lopez-Bote 27 ).
To increase the incorporation of PF in aquafeeds, particularly in marine species, further knowledge is needed on how dietary FA composition influences lipid metabolism. It is also required to understand the potential interactions between dietary carbohydrate and lipid source (LS), and how it may affect the regulation of processes involved in lipid metabolism.
Regardless of the lack of a dietary requirement of cholesterol in fish, it is an essential molecule with important cell membrane functions, and it is also the precursor of many physiologically active compounds (such as bile acids, vitamin D, adrenal corticoids and sex hormones)( 2 ).
The metabolic pathway of cholesterol biosynthesis, particularly in the liver, was transcriptionally up-regulated by VO feeding in salmonids( Reference Leaver, Villeneuve and Obach 4 ). In contrast to n-3 LC-PUFA content, tissue cholesterol levels were unaffected, meaning that a lower intake of dietary cholesterol in fish fed VO diets was fully compensated by increased cholesterol synthesis( Reference Leaver, Villeneuve and Obach 4 ).
The aim of this study was to evaluate whether an interaction between dietary LS and dietary carbohydrate level induced liver enzymatic activity and expression of genes related to lipid metabolism, particularly LC-PUFA and cholesterol pathways, in juveniles of a marine fish species, the European seabass.
Methods
Experimental diets
Four diets meeting the nutrient requirements of European sea bass( 2 ) but differing in carbohydrate content (0 and 20 % gelatinised starch, CH– and CH+, respectively) and LS (FO or VO) were formulated (Table 1). The dietary carbohydrate content was increased at the expense of dietary protein. In the CH– diets protein content was increased to replace carbohydrates. The VO was a blend of rapeseed (20 %), linseed (50 %) and palm (30 %) oils, and replaced circa 70 % of dietary lipids of the FO diets, which were provided by cod liver oil and FM.
Table 1 Ingredient and chemical composition of the experimental diets
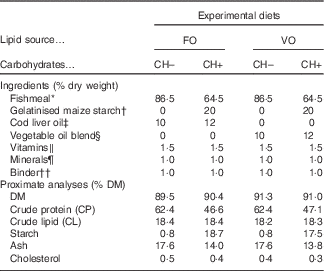
FO, fish oil; VO, blend of vegetable oils; carbohydrate content, 0 % (CH–) or 20 % (CH+) gelatinised maize starch.
* Steam-dried low temperature fishmeal; Pesquera Diamante (CP: 71·1 % DM; CL: 8·8 % DM).
† C-Gel Instant-12018; Cerestar.
‡ Labchem; Laborspirit Lda.
§ 30 % palm oil (Colmi), 50 % linseed oil (Sociedade Portuense de Drogas) and 20 % rapeseed oil (Huilerie Emile Noël SAS).
∥ Vitamins (mg/kg diet): retinyl acetate, 18 000 IU (6.19 mg)/kg diet; cholecalciferol, 2000 IU (0.04 mg)/kg diet; α tocopherol acetate, 35; sodium menadione bisulphate, 10; thiamin-HCl, 15; riboflavin, 25; calcium pantothenate, 50; nicotinic acid, 200; pyridoxine HCl, 5; folic acid, 10; cyanocobalamin, 0·02; biotin, 1·5; ascorbic acid, 50; inositol, 400 (Premix).
¶ Minerals (mg/kg diet): cobalt sulphate, 1·91; copper sulphate, 19·6; iron sulphate, 200; sodium fluoride, 2·21; potassium iodide, 0·78; magnesium oxide, 830; manganese oxide, 26; sodiumselenite, 0·66; zinc oxide, 37·5; dibasic calcium phosphate, 5·93 (g/kg diet); potassium chloride, 1·15 (g/kg diet); sodium chloride, 0·40 (g/kg diet) (Premix).
†† Aquacube (Guar gum, polymethyl carbamide, Manioc starch blend, hydrate calcium sulphate) (Agil).
All ingredients were finely ground, well mixed and dry pelleted in a laboratory pellet mill (California Pellet Mill), through a 3-mm die. The pellets were air-dried for 24 h and stored in a refrigerator (4ºC) until use.
Animals, experimental conditions and sampling
This experiment was directed by trained scientists (following FELASA category C recommendations) and conducted according to the European Union Directive (2010/63/EU) on the protection of animals for scientific purposes. The study was performed at the experimental facilities of the Marine Zoological Station, University of Porto, Portugal, in a thermo-regulated recirculation water system equipped with twelve fibreglass cylindrical tanks of 300 litre water capacity and supplied with continuous flow of filtered seawater. After 2 weeks of adaptation to the experimental conditions, twelve groups of twenty European sea bass (Dicentrarchus labrax) juveniles (initial body weight: 74·0 (sem 1·5) g) were established and randomly distributed into the tanks. At the beginning of the trial, fifteen fish from the stock population were sampled and pooled for whole-body composition analysis. The experimental diets were randomly assigned to triplicate groups of fish. During the trial, salinity averaged 35 (sem 1·0) g/l, dissolved oxygen was kept near saturation, and water temperature was regulated to 25·4 (sem 0·5)°C. The growth trial lasted 73 d, and during this period fish were hand-fed twice a day, 6 d a week, to apparent visual satiety. At the end of the trial, fish were unfed for 1 d to empty gut content and then bulk-weighed after mild anaesthesia with 0·3 ml/l methylethanol. To eliminate handling stress, fish continued to be fed for one more week and then, 18 h after the last meal (the previous day’s afternoon meal), nine fish from each tank were randomly sampled for blood, liver and muscle collection. Blood was collected without anaesthesia from the caudal vein using heparinised syringes and centrifuged at 2500 g for 10 min, and the recovered plasma was kept at –20ºC until analysis. Thereafter, the fish were killed with a sharp blow to the head, and whole body, viscera and liver were weighed for determination of hepatosomatic index and viscerosomatic index (VSI). Liver and muscle sections were frozen in liquid N2 and then stored at –80ºC until biochemical, enzymatic and molecular analyses.
Diets, whole fish, liver, muscle and plasma analysis
Chemical analysis of experimental diets, whole fish, liver and muscle was conducted according to the following procedures: DM after drying at 105°C until constant weight; ash by incineration in a muffle furnace at 450°C for 16 h; protein content (N×6·25) by the Kjeldahl method after acid digestion using a Kjeltec digestion and distillation unit (models 1015 and 1026, Tecator Systems; Höganäs); starch according to Beutler( Reference Beutler 28 ); and lipid by petroleum ether extraction (Soxtec HT System; Höganäs). The hepatic and muscular glycogen contents were determined as described by Roehrig & Allred( Reference Roehrig and Allred 29 ). Lipids in liver and muscle were determined according to the method used by Folch et al.( Reference Folch, Lees and Sloane Stanley 30 ). FA methyl esters were prepared by acid-catalysed transmethylation of total lipids using boron trifluoride (BF3) in methanol (14 %) according to Santha & Ackman( Reference Santha and Ackman 31 ) and analysed by GC (Varian 3900; Varian) as described in the study by Castro et al.( Reference Castro, Corraze and Panserat 32 ). Total cholesterol in the diets and tissues (liver and muscle) was assayed on total lipid extract by means of the Liebermann–Burchard method( Reference Stadtman 33 ).
Plasma metabolites were analysed using commercial kits from Spinreact: glucose (ref: 1001191), TAG (ref: 1001312), total cholesterol (ref: 1001090) and phospholipids (PL; ref: 1001140).
Enzymatic activity assays
The activity of key lipogenesis enzymes was determined in the liver. Liver was homogenised (dilution 1:4) in ice-cold buffer (100 mm-Tris-HCl, 0·1 mm-EDTA and 0·1 % triton X-100 (v/v), pH 7·8). All procedures were performed on ice. Homogenates were centrifuged at 30 000 g for 30 min at 4°C. After centrifugation, the resultant supernatant was collected and aliquots were stored at –80°C until analysis. All enzyme activities were measured at 37ºC, monitoring the changes in absorbance of NADPH at 340 nm in a microplate reader (ELx808™; BioTek Instruments), using 6·22 mm/cm as the millimolar extinction coefficient used for NADPH. The optimal substrate and protein concentrations for measurement of each enzyme activity were established by preliminary assays. Assay conditions were as follows.
Glucose-6-phosphate dehydrogenase (G6PDH; EC 1.1.1.49) activity was assayed as previously described by Morales et al.( Reference Morales, García-Rejón and De la Higuera 34 ), using a reaction mixture containing 50 mm-imidazole–HCl buffer (pH 7·4), 5 mm-MgCl2, 2 mm-NADP and 1 mm-glucose-6-phosphate.
Malic enzyme (ME; EC 1.1.1.40) activity was assayed using a reaction mixture containing 50 mm-imidazole–HCl buffer (pH 7·4), 5 mm-MgCl2, 0·4 mm-NADP and 2 mm-l-malate( Reference Singer, Mahadevappa and Ballantyne 35 ).
Fatty acid syntase (FAS; EC 2.3.1.38) activity was assayed as previously described by Chang et al.( Reference Chang, Seidman and Teebor 36 ), modified by Chakrabarty & Leveille( Reference Chakrabarty and Leveille 37 ). Samples were incubated with solution A (100 mm-potassium phosphate buffer pH 6·5, 0·1 mm-NADPH and 25 μm-acetyl-CoA) for 10 min. Then solution B (100 mm-potassium phosphate buffer pH 6·5 and 600 mm-malonyl-CoA) was added to this mixture.
Enzyme activities were expressed as milliunits per milligram of hepatic soluble protein (specific activity). Protein concentration was determined according to Bradford( Reference Bradford 38 ) using the Sigma protein assay kit and bovine serum albumin as standard. One unit of enzyme activity was defined as the amount of enzyme required to transform 1 μmol of substrate/min under the above assay conditions.
Gene expression analysis
Analyses of mRNA levels were performed on liver samples (two fish per tank). Total RNA was extracted using TRIzol reagent (Invitrogen) according to manufacturer recommendations, and RNA quality and quantity were assessed by gel electrophoresis and spectrophotometry (NanoDrop ND-1000; Nanodrop Labtech). Complementary DNA (cDNA) synthesis was performed with 1 µg of the resulting total RNA using the SuperScript III RNaseH-Reverse Transcriptase kit (Invitrogen) and random primers (Promega). Gene expression levels were determined by real-time quantitative PCR (q-PCR) using LightCycler® 480 II apparatus (Roche Diagnostics). Analyses were carried out using 2 µl of the diluted cDNA (1:76) mixed with 0·24 µl of each primer (10 µm), 3 µl LightCycler® 480 SYBR Green I Master (Roche Diagnostics GmbH) and 0·52 µl DNase/RNase/Protease-free water (5 prime GmbH) in a total volume of 6 µl. Primers were either found in the literature or designed to overlap an intron using Primer3 software( Reference Rozen and Skaletsky 39 ) and known sequences of European sea bass nucleotides in databases (Public Sigenae Contig Browser, Ensembl; http://public-contigbrowser.sigenae.org:9090/index.html) (Table 2).
Table 2 Sequences of the primer pairs used for real-time quantitative PCR determination of the transcript level of several European sea bass genes involved in hepatic and intestinal lipid and glucose metabolism

CYP3A27, cytochrome P450 3A27; CYP51A1, cytochrome P450 51 (lanosterol 14-α-demethylase); EF1α, elongation factor-1α; elovl5, elongase 5; –, no expression; FADS2, ∆6 fatty acyl desaturase; GLUT2, GLUT type 2; GK, glucokinase; G6Pase, glucose-6-phosphatase; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; LXR, liver X receptor; PEPCK, phosphoenolpyruvate carboxykinase; PK, pyruvate kinase; SREBP1, sterol response element binding protein-1.
* D Mazurais (unpublished results).
Thermal cycling was initiated with incubation at 95°C for 10 min for hot-start iTaq™DNA polymerase activation. A total of forty-five steps of PCR were then performed, each one consisting of heating at 95°C for 15 s for denaturing, at 60°C for 10 s for annealing and at 72°C for 15 s for extension. Following the PCR cycle, melting curves were systematically monitored (55°C temperature gradient at 0·5°C/5 s from 55 to 94°C) to ensure that only one fragment was amplified. Each PCR run included duplicates of reverse transcription for each sample and negative controls (RT-free samples, RNA-free samples). The PCR run for the reference gene included quadruplicates for each sample (duplicates of reverse transcription and PCR amplification, respectively) and negative controls. Quantification of the target gene transcripts in the liver was done using the elongation factor-1α (EF1α) gene expression as reference, as previously used in European sea bass by Geay et al.( Reference Geay, Santigosa and Culi 40 ) and that was stably expressed in the present study (data not shown). Relative quantification of the target gene transcript with the EF1α reference gene transcript was performed using the mathematical model described by Pfaffl( Reference Rozen and Skaletsky 39 ). The relative expression ratio (R) of a target gene was calculated on the basis of real-time PCR efficiency (E) and the CT deviation (ΔCT) of the unknown sample compared with a control sample and expressed in comparison with the EF1α reference gene:
Efficiency of q-PCR was measured by the slope of a standard curve using serial dilutions of cDNA.
Statistical analysis
Data were checked for normal distribution and homogeneity of variances and when appropriate were normalised. Statistical evaluation of data was carried out by a 2×2 factorial arrangement of treatments in a completely randomised experimental design (two-way ANOVA) with carbohydrate level and LS as fixed factors. The significance level of 0·05 was used for rejection of the null hypothesis. In cases where interaction was significant, one-way ANOVA was performed for each factor. All statistical analyses were conducted using the SPSS 21.0 software package (IBM Corp.) for Windows.
Results
Dietary fatty acid composition
The four diets presented small differences in the proportions of total SFA (slightly higher in VO diets) and MUFA(higher in FO diets) (Table 3). Within MUFA, high levels of oleic acid (18 : 1n-9) were found in VO diets, whereas the opposite was seen for palmitoleic acid (16 : 1n-7), eicosenoic acid (20 : 1n-9) and erucic acid (22 : 1n-9). Linoleic acid (18 : 2n-6) was strongly higher in VO diets than in FO diets. Regarding n-3 PUFA, VO diets were particularly rich in linolenic acid (18 : 3n-3) and poor in EPA and DHA.
Table 3 Fatty acid composition (% of total fatty acids) of the experimental diets

FO, fish oil, VO, blend of vegetable oils; carbohydrate content, 0 % (CH–) or 20 % (CH+) gelatinised maize starch.
Growth performance and feed utilisation
No mortality was observed during the trial. At the end of the feeding trial, there were no differences among dietary treatments on growth performance or feed utilisation (Table 4). Feed intake (gram per kilogram average body weight per d) was not affected by experimental diets, and therefore N intake was lower in the CH+ diet. N retention, expressed per unit weight gain, was not affected by diet composition, but when its retention was expressed as percentage of intake it was higher in the CH+ groups. Lipid retention, expressed as either percentage of lipid intake or per unit weight gain, increased with dietary carbohydrate intake (Table 4). No significant effect of LS or of interaction between carbohydrate and LS was observed for any of these parameters.
Table 4 Growth performance and feed utilisation of European seabass fed the experimental dietsFootnote † (Mean values with their standard errors; n 3)

Fish oil (FO), blend of vegetable oils (VO); CH content, 0 % (CH–) or 20 % (CH+) gelatinised maize starch; IBW, initial body weight; FWB, final body weight; ABW, average body weight.
* Significant differences at P<0·05 (two-way ANOVA).
† ABW: IBW+FBW/2.
‡ DGI: ((FBW1/3−IBW1/3)/time in d)×100.
§ FE, wet weight gain/dry feed intake.
∥ PER, wet weight gain/crude protein intake.
¶ N retention=((FBW×carcass N content)−(IBW×carcass N content))/(ABW×number of d).
†† N retention=N retention (g/kg ABW/d)/N intake.
‡‡ Lipid retention=((FBW×carcass lipid content)−(IBW×carcass lipid content))/(ABW×number of d).
§§ Lipid retention=lipid retention (g/kg ABW/d)/lipid intake.
Whole-body, liver and muscle composition
Whole-body protein and ash contents were not affected by dietary treatments, but higher lipid content and hepatosomatic index and VSI were observed in the CH+ groups (Table 5). Glycogen content increased in the liver and muscle with the CH+ diet (Table 5). An increase in lipid content in the liver was also observed, but no differences were detected in muscle lipids, nor in cholesterol content, in either tissue.
Table 5 Whole-body, liver and muscle composition (wet-weight basis), hepatosomatic and viscerosomatic indices of European sea bass fed the experimental dietsFootnote † (Mean values with their standard errors)

FO, fish oil; VO, blend of vegetable oils; CH content, 0 % (CH–) or 20 % (CH+) gelatinised maize starch.
* Significant differences at P<0·05 (two-way ANOVA).
† n 3, for whole body composition; n 6 for lipids and cholesterol; n 9 for glycogen; n 18 for hepatosomatic index (HSI) and viscerosomatic index (VSI).
‡ Initial body composition on the fish: DM, 27·47 %; protein, 15·26 %; lipid, 10·82 %; ash, 4·87 %.
§ HSI: (liver weight/body weight)×100.
∥ VSI: (viscera weight/body weight)×100.
Liver and muscle lipid, cholesterol and glycogen content were not affected by dietary LS (Table 5).
Liver and muscle fatty acid profiles
Muscle and liver FA composition was affected by diet composition and mirrored the FA profile of the dietary LS (Tables 6 and 7). Accordingly, the muscle FA profile of fish fed the VO diet was characterised by lower levels of SFA, higher levels of n-6 PUFA (particularly high 18 : 2n-6 levels) and n-3 PUFA (particularly high 18 : 3n-3 levels), and low arachidonic acid (20 : 4n-6), EPA and DHA. The CH+ diet also increased muscle SFA and promoted a decrease of n-3 PUFA (Table 7).
Table 6 Liver fatty acid profile (% of total fatty acids; FA) of European seabass fed the experimental dietsFootnote † (Mean values with their standard errors; n 6)

FO, fish oil; VO, blend of vegetable oils; CH content, 0 % (CH–) or 20 % (CH+) gelatinised maize starch; n-3 LC-PUFA, n-3 long chain PUFA.
* Significant differences at P<0·05 (two-way ANOVA).
† FA ≥0·02 %; <0·02 % was not considered in the table as it was below detection.
Table 7 Muscle fatty acid profile (expressed as % of total fatty acids; FA) of European seabass fed the experimental dietsFootnote † (Mean values with their standard errors; n 6)

FO, fish oil, VO, blend of vegetable oils; CH content, 0 % (CH–) or 20 % (CH+) gelatinised maize starch; n-3 LC-PUFA, n-3 long chain-PUFA.
* Significant differences at P<0·05 (two-way ANOVA).
† FA≥ 0·02 %; <0·02 % was not considered in the table.
Hepatic n-6 PUFA was higher in the VO groups than in the FO groups (particularly 18 : 2n-6, 18 : 3n-6 and 20 : 4n-6) and lower in groups fed the CH+ diet than in those fed the CH– diet (particularly 20 : 2n-6 and 20 : 4n-6 (Table 6)). Fish fed the CH+ diet also presented increased MUFA levels. In the liver, an interaction between dietary carbohydrate and LS was noticed in SFA and n-3 PUFA content. Thus, n-3 PUFA content in fish fed the CH+ diet slightly increased when FO was replaced by VO, whereas it considerably decreased in fish fed the CH– diet. Opposite results were observed for the SFA content, and this was essentially because of differences in palmitic acid levels.
Plasma metabolites
Dietary carbohydrate intake promoted an increase in plasma cholesterol and PL levels (Table 8), whereas dietary VO decreased plasma PL levels. No differences among dietary treatments were observed on plasma glucose and TAG levels.
Table 8 Plasma metabolites levels (mmol/l) in European seabass fed the experimental diets (Mean values with their standard errors; n 18)
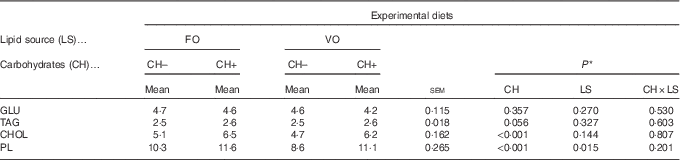
FO, fish oil; VO, blend of vegetable oils; CH content, 0 % (CH–) or 20 % (CH+) gelatinised maize starch; GLU, glucose; CHOL, total cholesterol; PL, phospholipids.
* Significant differences at P<0·05 (two-way ANOVA).
Enzymes activity
Replacement of FO by VO did not affect the activity of hepatic FAS, ME or G6PDH, which are key hepatic enzymes involved in lipogenesis (Table 9). On the other hand, dietary carbohydrate induced an increase in ME and G6PDH activities but not in FAS activity.
Table 9 Enzymatic activity (mU/mg protein) of selected enzymes involved in lipogenesis in European seabass fed the experimental diets (Mean values with their standard errors; n 9)
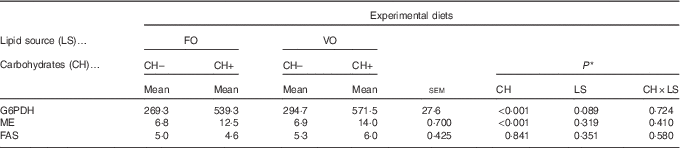
FO, fish oil; VO, blend of vegetable oils; CH content, 0 % (CH–) or 20 % (CH+) gelatinised maize starch; G6PDH, Glucose-6-phosphate dehydrogenase; ME, malic enzyme; FAS, fatty acid synthase.
* Significant differences at P<0·05 (two-way ANOVA).
Gene expression
In the liver, expression of glucokinase (GK), the first key glycolytic enzyme, and of phosphoenolpyruvate carboxykinase (PEPCK), the first key gluconeogenic enzyme, responded to diet composition, whereas pyruvate kinase (PK) and glucose 6-phosphatase (G6Pase), enzymes involved in the last step of glycolysis and gluconeogenesis, respectively, were not nutritionally regulated (Fig. 1). PEPCK mRNA levels were higher in fish fed the CH– diet and were not affected by dietary LS, whereas an interaction between carbohydrate level and LS was observed for GK transcripts. Accordingly, GK transcription was strongly induced by dietary carbohydrate (starch) intake, and it was also induced by VO intake, but only in fish fed the CH– diet. Liver GLUT type 2 (GLUT2), the protein involved in glucose transport, transcript levels were not affected by diet composition (Fig. 1).

Fig. 1 mRNA levels of proteins involved in glycolysis (GK, glucokinase; PK, pyruvate kinase), gluconeogenesis (PEPCK, phosphoenolpyruvate carboxykinase; G6Pase, glucose 6-phosphatase), and glucose transport (GLUT type 2, GLUT2) in the liver of European sea bass fed the experimental diets. Expression values are normalised by elongation factor-1α (EF1α)-expressed transcripts. Fish oil (FO), blend of vegetable oils (VO); carbohydrate content, 0 % (CH–) or 20 % (CH+) gelatinised maize starch. ![]() , CH–;
, CH–; ![]() , CH+. Values are means (n 6), with their standard errors represented by vertical bars. Significant differences at P<0·05 (two-way ANOVA).
, CH+. Values are means (n 6), with their standard errors represented by vertical bars. Significant differences at P<0·05 (two-way ANOVA).
Data on the expression marker genes encoding the proteins involved in cholesterol biosynthesis in the liver are shown in Fig. 2. Feeding VO diets up-regulated CYP51A (cytochrome P450 51) (lanosterol 14-α-demethylase) mRNA levels in the liver. Hepatic HMGCR (3-hydroxy-3-methylglutaryl-coenzyme A reductase) transcript pattern showed significant interaction between dietary carbohydrate level and LS in the liver. Induction of HMGCR mRNA levels was observed in fish fed the VOCH– diet. HMGCR transcript levels were also induced by dietary carbohydrate, but only in fish fed the FO diet. In the liver, dietary carbohydrate also promoted an increase of CYP3A27 (cytochrome P450 3A27) mRNA levels and a down-regulation of LXR (liver X receptor) transcription.

Fig. 2 mRNA levels of proteins involved in cholesterol biosynthesis (HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; CYP51A1, cytochrome P450 51 (lanosterol 14-α-demethylase); CYP3A27, cytochrome P450 3A27; LXR, liver X receptor) in the liver of European sea bass fed the experimental diets. Expression values are normalised by elongation factor-1α (EF1α)-expressed transcripts. Fish oil (FO), blend of vegetable oils (VO); carbohydrate content, 0 % (CH–) or 20 % (CH+) gelatinised maize starch. ![]() , CH–;
, CH–; ![]() , CH+. Values are means (n 6), with their standard errors represented by vertical bars. Significant differences at P<0·05 (two-way ANOVA).
, CH+. Values are means (n 6), with their standard errors represented by vertical bars. Significant differences at P<0·05 (two-way ANOVA).
Liver expression of FADS2 (∆6 fatty acyl desaturase), a protein involved in the LC-PUFA biosynthesis pathway, and of SREBP1 (sterol regulatory element binding protein 1) were not affected by diet composition (Fig. 3). We were unable to detect expression of hepatic elongase 5 (elovl5) in the liver.

Fig. 3 mRNA levels of proteins involved in LC-PUFA biosynthesis (FADS2, ∆6 fatty acyl desaturase; SREBP1, sterol response element binding protein-1) in the liver of European sea bass fed the experimental diets. Fish oil (FO), blend of vegetable oils (VO); carbohydrate content, 0 % (CH–) or 20 % (CH+) gelatinised maize starch. Expression values are normalised by elongation factor-1α (EF1α)-expressed transcripts. ![]() , CH–;
, CH–; ![]() , CH+. Values are means (n 6), with their standard errors represented by vertical bars. Significant differences at P<0·05 (two-way ANOVA).
, CH+. Values are means (n 6), with their standard errors represented by vertical bars. Significant differences at P<0·05 (two-way ANOVA).
Discussion
Although an effect of dietary protein level on the parameters analysed in this study cannot be completely discarded, protein requirements of European sea bass were fulfilled in all dietary treatments, and we assume that dietary carbohydrate level and LS were the main factors responsible for the observed effects.
Effect of dietary carbohydrate
As previously observed by Peres & Oliva-Teles( Reference Peres and Oliva-Teles 42 ), in the present study reduction of dietary protein from 60 to 45 % by increasing gelatinised starch content did not affect growth performance. N retention (% N intake) was also higher in the CH+ groups, further supporting the protein-sparing effect of dietary starch.
The HSI in the CH+ groups is in agreement with the higher glycogen and lipid levels, and it is also in accordance with previous data on this species( Reference Peres and Oliva-Teles 42 ) and other fish species( Reference Kamalam, Medale and Kaushik 24 , Reference Couto, Enes and Peres 43 , Reference Coutinho, Peres and Guerreiro 44 ). Dietary carbohydrate also promoted higher whole-body lipid retention, and most of it was deposited in the viscera, as reflected in the positive correlation between VSI and dietary starch. Such a relationship was also previously reported in European sea bass by Peres & Oliva-Teles( Reference Peres and Oliva-Teles 42 ). In the muscle, an increase in glycogen content but not in lipid content was also observed in fish fed the CH+ diet.
Fish, particularly carnivorous fish, have limited ability to use dietary carbohydrate, and a persistent hyperglycaemia is observed in several species after administration of glucose or a carbohydrate-rich diet( Reference Enes, Peres and Sanchez-Gurmaches 23 , Reference Polakof, Panserat and Soengas 45 ). European sea bass is, however, able to restore basal plasma glucose levels within 12 h after feeding, and present data on glucose levels 18 h after feeding are in accordance with such previous evidence( Reference Peres, Goncalves and Oliva-Teles 22 , Reference Castro, Corraze and Panserat 32 ). In the liver, excess dietary glucose is converted to glycogen or lipids or burned for energy purposes. Glucose can also enter the pentose phosphate pathway, yielding reducing power (NADPH) for biosynthesis of FA and cholesterol( Reference Coutinho, Peres and Guerreiro 44 ). In the present study, hepatic transcriptional regulation of rate-limiting glycolytic and gluconeogenic enzymes by dietary carbohydrate in European sea bass was also observed( Reference Enes, Peres and Sanchez-Gurmaches 23 , Reference Gatesoupe, Huelvan and Le Bayon 47 ). Accordingly, gene expression of GK, the first enzyme involved in the glycolytic pathway, was induced by dietary carbohydrate, whereas expression of PEPCK, the first enzyme of gluconeogenesis, was depressed by dietary carbohydrate. Transcriptional regulation of GK by dietary carbohydrate intake was at first observed in European sea bass by Enes et al.( Reference Enes, Panserat and Kaushik 48 ). Lack of transcriptional regulation of PK by dietary carbohydrate is possibly linked to a post-transcriptional mechanism, as Enes et al.( Reference Enes, Panserat and Kaushik 48 ) have shown that PK activity was induced by dietary carbohydrate intake. In European sea bass, modulation of PEPCK activity by dietary carbohydrates was not evaluated( Reference Enes, Peres and Sanchez-Gurmaches 23 ). However, hepatic activity of other key gluconeogenic enzymes, fructose-1,6-bisphosphatase (FBPase) and G6Pase, revealed a lack of regulation by dietary carbohydrate( Reference Enes, Panserat and Kaushik 48 ). In this study, we also observed no transcriptional regulation of G6Pase, but PEPCK was down-regulated by dietary carbohydrate. Together, these results indicate that carbohydrate catabolism seems to be adequately regulated at nutritional level in European sea bass. This is in agreement with the conclusion of Enes et al.( Reference Enes, Panserat and Kaushik 49 ) that the activity of key enzymes of the glycolytic pathway is enhanced by dietary carbohydrate. On the other hand, available data on regulation of the gluconeogenic pathway by dietary carbohydrate are still not consistent. According to Enes et al.( Reference Enes, Panserat and Kaushik 49 ) an inverse regulation of the gluconeogenic enzyme activities with dietary carbohydrate intake is not observed in European sea bass and gilthead sea bream (Sparus aurata), but the present results on PEPCK suggest that gluconeogenesis is in part regulated at the transcriptional level.
Increased whole-body lipids, hepatic and muscular glycogen levels and hepatic lipids (mainly 16 : 0) in fish fed the CH+ diet indicate that both glycogenesis and lipogenesis constitute important routes for the excess circulating glucose, as described in this species( Reference Moreira, Peres and Couto 50 ) and other fish species( Reference Fernández, Miquel and Córdoba 51 , Reference Figueiredo-Silva, Saravanan and Schrama 52 ). Present data on the activity of enzymes G6PDH and ME, which are the main providers of NADPH required for lipogenesis, partially corroborate these observations. On the other hand, no changes on FAS activity were observed, despite the increased hepatic accumulation of 16 : 0, the final product of FAS activity( Reference Leaver, Bautista and Bjornsson 53 , Reference Engelking 54 ). As 16 : 0 exerts a negative feedback on FAS activity, this may explain the lack of variation in FAS activity. Similarly, Dias et al.( Reference Dias, Alvarez and Diez 21 ) also found no correlation between starch intake and FAS activity in European sea bass.
Interestingly, dietary carbohydrate intake promoted an increase in cholesterol levels in the plasma but not in the liver. An increase in plasma cholesterol concentration was also recorded in rabbits and monkeys( Reference Kritchevskv and Tepper 55 – Reference Srinivasan, Radhakrishnamurthy and Webber 57 ) and in rainbow trout( Reference Kamalam, Médale and Larroquet 25 ) fed carbohydrate-rich diets. Besides hypercholesterolaemia, dietary carbohydrate also induces alterations of plasma lipoprotein profile, including increased levels of VLDL and LDL( Reference Sparks, Sparks and Kritchevsky 56 , Reference Srinivasan, Radhakrishnamurthy and Webber 57 ). Increased VLDL may be related to an inductor effect of carbohydrate on cholesterol synthesis. Accordingly, in the present study, dietary carbohydrate induced hepatic up-regulation of HMGCR transcript levels, a key protein involved in cholesterol biosynthesis.
Cholesterol biosynthesis is an energy-demanding process and also requires high amounts of NADPH( 2 , Reference Norambuena, Lewis and Hamid 58 ), which are mainly obtained through G6PDH activity( Reference Richard, Mourente and Kaushik 10 ). Thus, in the present trial, the increased activity of G6PDH observed in the CH+ groups might be at least partially related to cholesterol biosynthesis and not to FA synthesis. However, there were no differences on hepatic cholesterol content among dietary groups.
Transcript levels of CYP3A27 were also increased in fish fed the carbohydrate-rich diet. This enzyme belongs to a superfamily of cytochrome P450 (CYP) haem containing mono-oxygenases involved in oxidative metabolism of many xenobiotics( Reference Pikuleva 59 , Reference Uno, Ishizuka and Itakura 60 ). Similarities between CYP3A27 and human CYP3A4 were described in rainbow trout( Reference Lee and Buhler 61 ). CYP3A4 participates in the upstream steps of one of a number of pathways involved in cholesterol catabolism. Specifically, it is responsible for the conversion of cholesterol to 4β-hydroxycholesterol before its elimination in bile salts. However, considering that the 4β-hydroxycholesterol formation rate is very slow, it is believed that CYP3A4 may play a greater role in the transcriptional regulation of lipid metabolism than in cholesterol elimination( Reference Pikuleva 59 ). In fact, the endogenous oxidised cholesterol derivate oxysterol 4β-hydroxycholesterol is described to activate the nuclear receptor LXR( Reference Pikuleva 59 , Reference Zhao and Dahlman-Wright 62 ). This nuclear receptor, besides its role in activation of transcription of target genes that protect cells from cholesterol overload( Reference Zhao and Dahlman-Wright 62 ), also activates lipogenic genes and regulate the transcription of genes involved in glucose metabolism( Reference Mitro, Mak and Vargas 63 ), either alone or in conjunction with other nutrient-sensing transcription factors such as SREBP1 ( Reference Grønning-Wang, Bindesbøll and Nebb 64 ). However, contradictory to what was expected, there was a down-regulation of hepatic X receptor (LXR) gene expression in fish fed a carbohydrate-rich diet, although the effect was very small (P=0·048). Direct regulation of SREBP1 by LXR was previously described( Reference Grønning-Wang, Bindesbøll and Nebb 64 ), although in the present study such modulation was not observed, which may be related to the small differences in LXR expression among groups.
The inductor effect of dietary carbohydrate on the LC-PUFA biosynthesis pathway through increased transcript levels of elovl5 and FADS2 that was reported in salmonids( Reference Seiliez, Panserat and Kaushik 17 , Reference Kamalam, Médale and Larroquet 25 ) was not observed in the present study. On the contrary, the proportion of n-3 LC-PUFA, particularly EPA and DHA, decreased in the liver of fish fed a carbohydrate-rich diet.
Although PL biosynthesis pathway regulation or tissue PL content was not evaluated, the higher plasma PL levels observed in the CH+ groups suggest that dietary carbohydrate may also induce PL biosynthesis. Further studies are, however, required to confirm this assumption.
Effect of lipid source
Similar to previous results in this species( Reference Richard, Mourente and Kaushik 10 , Reference Mourente, Dick and Bell 65 – Reference Figueiredo-Silva, Rocha and Dias 67 ), no effects on growth parameters and feed utilisation of European sea bass were observed due to dietary FO replacement by VO blend. On the other hand, the well-established effect of FO replacement by VO at tissue FA compositional level, such as increased C18 FA (18 : 3n-3; 18 : 2 n-6) and decreased levels of LC-PUFA (EPA and DHA), was also observed in this study.
Contrary to humans and other mammals, in which LC-PUFA of FO reduce triglyceridaemia and lipogenesis( Reference Geelen, Schoots and Bijleveld 68 – Reference Harris, Miller and Tighe 71 ), in fish such effects are not clear. Studies in fish showed that FO either depressed( Reference Jordal, Lie and Torstensen 15 ), had no effects( Reference Richard, Kaushik and Larroquet 9 , Reference Richard, Mourente and Kaushik 10 , Reference Regost, Arzel and Robin 12 , Reference Torstensen, Froyland and Lie 14 ) or had opposite effects( Reference Menoyo, Izquierdo and Robaina 13 ) on lipogenesis.
Contrary to our previous study in European sea bass fed a similar VO blend( Reference Castro, Corraze and Panserat 32 ) or plant-based diets( Reference Geay, Ferraresso and Zambonino-Infante 6 , Reference Geay, Santigosa and Culi 40 ), in the present study no up-regulation of FADS2 was observed at hepatic level in fish fed the VO diets. Also contrary to previous observations( Reference Castro, Corraze and Panserat 32 ), we were unable to detect the expression of elovl5 in the liver. The sampling time in this trial (18 h post-feeding) may explain the apparent lack of nutritional regulation of FADS2 as by that time almost all ingested feed had been already digested and metabolically processed.
Previous studies in rainbow trout and gilthead sea bream reported higher specificities of C18 PUFA (which are characteristic of VO) to be reacylated into TAG, whereas LC-PUFA are reacylated into PL( Reference Pérez, Rodríguez and Henderson 72 , Reference Caballero, Izquierdo and Kjørsvik 73 ). It is therefore possible that similar processes may have happened in fish fed the VO-based diets, thus leading to the decrease in plasma PL levels in the present study.
Regulation of cholesterol biosynthesis is mainly controlled by the rate-limiting enzyme HMGCR by a feedback mechanism( Reference Engelking 54 ). In the present study, we observed an up-regulation of this enzyme at hepatic level in the VO groups. In European sea bass fed plant-based diets, an up-regulation of hepatic transcript levels of HMGCR was also previously observed( Reference Geay, Ferraresso and Zambonino-Infante 6 ). We also observed increased mRNA levels of CYP51A1, an enzyme involved in the last steps of cholesterol synthesis, specifically in the serial reactions that convert lanosterol to cholesterol( Reference Nelson and Cox 46 , Reference Liscum 74 ). Similarly, Leaver et al.( Reference Leaver, Villeneuve and Obach 4 ) and Geay et al.( Reference Geay, Ferraresso and Zambonino-Infante 6 ) also observed an up-regulation of CYP51A1 transcript levels in Atlantic salmon fed VO-based diets and in European sea bass fed PF-based diets. In Atlantic salmon fed plant-based diets increased hepatic cholesterol biosynthesis and impaired intestinal cholesterol absorption were found( Reference Liland, Espe and Rosenlund 3 , Reference KortnerTM and Krogdahl 75 ). Phytosterols and/or PF were advanced as the possible causes for the reduced intestinal cholesterol absorption. In our study, expression of genes encoding for cholesterol uptake was not assessed. With dietary cholesterol levels being similar among groups fed FO and VO, we can speculate that in the present study VO diets also promoted a reduction of intestinal cholesterol absorption, and this might have induced endogenous production of cholesterol in the liver to counter-balance the absorbed cholesterol deficit.
Carbohydrate and lipid source interaction
Contrary to previous evidence on the inductor role of carbohydrates on the LC-PUFA biosynthesis pathway of salmonids( Reference Seiliez, Panserat and Kaushik 17 , Reference Kamalam, Médale and Larroquet 25 ), we failed to find a potential interactive effect of dietary LS and carbohydrate on the transcriptional regulation of LC-PUFA biosynthesis.
At the organ compositional level, in the liver an interaction between dietary carbohydrate and LS on total hepatic n-3 PUFA, but not for LC-n-3-PUFA, was detected. Within the CH– group, total n-3 PUFA content was higher in the FOCH– group, whereas within the CH+ groups no differences on total n-3 PUFA between VO and FO groups were observed. An interaction was also observed on the transcript levels of HMGCR, as differences were only noticed within CH– and FO groups. These results suggest that, although both VO and carbohydrates seem to have an inductor effect on transcript levels of HMGCR, they do not act synergistically.
Among analysed actors involved in carbohydrate metabolism, only transcript levels of GK in the liver were modulated by dietary LS. Liver interaction of GK transcript levels showed a pattern identical to that described for HMGCR. Although the recognised on–off regulation of GK at the transcriptional level in response to dietary carbohydrate was evident in the FO groups, this effect was not clear within VO groups. In the CH– groups, GK mRNA levels were considerably higher in the VO than in the FO group. Recent studies in salmonids suggest that replacement of marine fishery-derived feedstuffs by plant products affects hepatic carbohydrate metabolism( Reference Panserat, Hortopan and Plagnes-Juan 5 , Reference Geay, Ferraresso and Zambonino-Infante 6 , Reference Morais, Pratoomyot and Taggart 76 ). Although the present results may suggest an induction of glycolysis by VO in the CH– groups, care must be taken in the interpretation of data because GK gene expression was very low in the CH– groups and may not be biologically significant.
Conclusions
In this study no key interactions between dietary LS and carbohydrates were detected in European sea bass juveniles. An inductor role of carbohydrates in the LC-PUFA biosynthesis pathway at the transcriptional level previously observed in salmonids was also not observed in European sea bass juveniles.
This study describes for the first time a direct relation between dietary carbohydrate and the cholesterol biosynthetic pathway in fish. The present results seem to be promising, considering that dietary supply of cholesterol and PL will be limited in future aquafeeds with the replacement of both FM and FO by PF and VO. However, further insights into the regulation of cholesterol metabolism are needed, particularly in specific stages of life as reproduction and larvae phases.
Acknowledgements
The authors express their thanks to P. Correia for technical assistance.
This work was partially supported by the FCT (Foundation for Science and Technology), Portugal (project PTDC/MAR-BIO/4107/2012) and co-financed by the European Regional Development Fund (ERDF) through the COMPETE – Operational Competitiveness Programme and national funds through FCT – under the project ‘PEst-C/MAR/LA0015/2011’. C. C. was supported by a grant (SFRH/BD/76297/2011) and A. P. J. (SFRH/BPD/64684/2009) from FCT, Portugal.
C. C. carried out the main experimental work and wrote the draft of the manuscript under the direction of the project designer and leaders A. O. T., G. C. and S. P. A. P. J. assisted with the biochemical analyses and draft writing. L. L. performed the fatty acid analyses. M. C. assisted with the gene expression analyses. All authors contributed to and approved the manuscript.
The authors declare that there are no conflicts of interest.

















