Conventionally, proximal behaviours are thought to strongly modify disease risk but examining early life factors may yield intriguing insights into the origins of later disease( Reference Barker 1 ). For example, clarifying how the infant diet may influence later obesity risk is important as it may highlight a window of opportunity for designing interventions that promote health across the life course. In particular, the role of dietary protein in modifying obesity risk deserves further attention( Reference Simpson and Raubenheimer 2 ).
During infancy, adequate protein intake is critical to growth and development( 3 ). Deficiencies in protein and/or other macronutrients result in failure to attain age-appropriate height and weight( Reference Brabin and Coulter 4 ). In resource-poor settings, diets limited in high-quality or animal protein exacerbate risk of undernutrition( Reference Michaelsen, Larnkjær and Mølgaard 5 ). The role of protein in reducing undernutrition is well established; however, studies from the Developmental Origins of Health and Disease (DOHaD) perspective suggest that infant protein intake may promote later obesity via its effects on adipocyte differentiation and adipogenesis( Reference Koletzko, Broekaert and Demmelmair 6 ) and the timing of the adiposity rebound( Reference Rolland-Cachera, Deheeger and Maillot 7 , Reference Rolland-Cachera, Deheeger and Akrout 8 ). In fact, studies from higher-income settings have found independent associations of infant protein intake with later obesity( Reference Michaelsen, Larnkjær and Mølgaard 5 ). Reviews of these studies acknowledge convincing evidence( Reference Hörnell, Lagström and Lande 9 ) that protein intake in early life is positively associated with risk of obesity, but the mechanisms are yet to be confirmed( Reference Lind, Larnkjaer and Molgaard 10 ).
Whereas protein intake in early life may have these long-term effects on later risk of obesity, the role of proximal protein intake (in adulthood) merits attention as well, as adulthood protein intake also contributes to adulthood obesity risk. Proteins may exert beneficial effects on adult metabolic risk by promoting satiety (thus reducing overeating), increasing thermogenesis (which promotes fat metabolism) and improving glucose metabolism( Reference Westerterp-Plantenga 11 , Reference Pesta and Samuel 12 ). In fact, the ‘protein leveraging’ hypothesis stresses that the satiating effects of protein strongly regulate total energy intake, and thus, modifying dietary protein may be one approach to curb the obesity epidemic( Reference Simpson and Raubenheimer 2 , Reference Simpson and Batley 13 ). ‘Nutrition transition’ refers to the increased consumption of refined carbohydrates and fats that has occurred in recent decades; this transition has diversified diets in several low- and middle- income countries( Reference Drewnowski and Popkin 14 – Reference Popkin, Adair and Ng 17 ). Proponents of the protein leveraging hypothesis suggest that the reduced protein-density of these diversified diets may induce overeating to meet daily protein requirements( Reference Simpson and Raubenheimer 2 , Reference Simpson and Batley 13 ).
Although a review of the literature yields these important examples of how protein intake may modify obesity risk in an age-dependent manner, no study has fully accounted for the role of dietary protein patterns in modifying obesity risk. When studies focus on protein intakes in distinct time periods, this approach neglects the potential roles of cumulative protein intake patterns across the life course. In light of the rapid westernisation of traditional diets worldwide( Reference Popkin, Adair and Ng 17 ), an individual may experience periods of excess, adequate and insufficient protein intake in a single lifespan( Reference Abdullah 18 , Reference Tzioumis and Adair 19 ). It is unclear how combinations of such exposures across the life course may influence adult BMI. Moreover, outside of infancy, other sensitive periods may exist but are yet to be identified; hence, studying pubescence or adolescence could also grant important insights( Reference Schooling 20 , Reference Viner, Ross and Hardy 21 ). Thus, we employed a life-course approach( Reference Darnton-Hill, Nishida and James 22 , Reference Ben-Shlomo and Kuh 23 ) to analyse how protein intake affects later body composition. We asked two main questions. First, are there periods of the life course during which protein intake may modify later BMI, fat mass and lean mass? To this end, we estimated how protein intake in early childhood, pubescence, adolescence and young adulthood was associated with later BMI, fat mass and lean mass. We expected that protein intake in early childhood would be positively associated with adulthood BMI and that protein intakes at later ages would be inversely associated with adult BMI, fat mass and lean mass. Second, does life history of protein intake differentially relate to later BMI? We explored how histories of protein intake related to body size in young adulthood. We hypothesised that there would be distinct trajectories of protein intake, and that these trajectories would be differentially associated with BMI, fat mass and lean mass in young adulthood.
Methods
Study population
The Cebu Longitudinal Health and Nutrition Survey (CLHNS) follows a birth cohort of infants born in Cebu, Philippines( Reference Adair, Hindin and Popkin 24 ). Detailed dietary and anthropometric data were collected at bimonthly intervals between birth and 24 months (1983–1986), and in five subsequent post-infancy surveys: 1991–1992, 1994–1995, 1998–1999, 2002 and 2005( Reference Adair, Hindin and Popkin 24 ). There were 3080 singleton infants at birth and 1885 individuals in 2005.
Diet
Diet was assessed using 24 h dietary recalls, one recall for each bimonthly survey from birth to 24 months and two recalls for surveys from 11 years onwards. The means of the dietary intakes reported at 22 and 24 months were used to estimate usual intake at age 2 years. Protein intake was estimated using the Filipino Food Composition Tables ( 25 ). Nutrients from breastmilk were not quantified; thus, dietary data at 22 and 24 months only account for estimated nutrient intake from complementary foods and underestimate total nutrient intake in 14 % infants that were still being breastfed. Analyses presented here include surveys that collected recall data from age 22 and 24 months and 11, 15, 19 and 22 years. The 1991 survey employed an FFQ which systematically overestimated macronutrient intake data and so it was omitted from these analyses.
Primary exposure
The primary exposure was ‘protein relative to needs’; this was the difference between estimated protein intake and the age-specific recommended daily allowance (RDA) of protein (g/kg body weight). An absolute specification of protein intake would have been inappropriate and misleading as heavier people have greater energy needs, and thus, tend to consume more macronutrients. Our specification of protein intake (g/kg body weight) more appropriately conveys whether a surplus (if positive) or deficit (if negative) of protein intake was consumed relative to individual needs. RDA for years 2, 11, 15, 19 and 22 were 1·05, 0·95, 0·85, 0·80 and 0·80 g/kg body weight, respectively( 3 ).
Confounders and key covariates
Statistical models were adjusted for variables that might potentially confound the association of protein intake with later BMI, lean mass and fat mass, given their hypothesised associations with both protein intake and body composition. Models also included variables that were strongly associated with body composition at age 22 years to clarify whether observed associations between protein intake trajectories and body composition were independent of these variables. We included sex, factors from the time of the offspring’s birth (offspring weight (kg), maternal education (years) and maternal height (cm)), factors from early childhood (duration of breast-feeding (months), a household assets score at age 2 years and offspring BMI (kg/m2) at age 2 years) and variables related to the offspring at age 22 years (household assets, physical activity level, education (years), energy intake (4184 kJ (1000 kcal) units) and residuals of carbohydrate and fat intake). The composite score of household assets ranged from 0 to 11 and was based on individual possession of the following: electricity, house, material of house, air conditioner, television, tape recorder, refrigerator, electric fan, jeepneys (customised taxi jeeps), car and clothing iron. Physical activity at age 22 years was a binary variable based on normal (600–1000 metabolic equivalent minutes (METS)/week) or low (<600 METS/week) reported activity, following the WHO recommendations( 26 ). We did not include time-varying factors for interim years (ages 11, 15 or 19 years) either because they were unavailable (physical activity), were presumably on the pathway between protein intake and early adulthood BMI (interim BMI or macronutrients) or had negligible effects on regression coefficients when included (interim asset scores).
Primary outcome
BMI (kg/m2) at age 22 years was the primary outcome. Lean mass (kg) and fat mass (kg) at age 22 years were secondary outcomes. Weight, height and skinfold thicknesses were measured by trained personnel. Body fat percentage was calculated from skinfold thicknesses using equations validated in Asian populations( Reference Deurenberg and Deurenberg-Yap 27 ). Fat mass (kg) was then calculated as %body fat×weight (kg); lean mass (kg) was the difference between body weight and fat mass. All three outcomes were stratified by sex and then standardised to the mean for males and females in the sample to aid in interpretability. The kg or kg/m2 equivalents are provided in-text for context. Pregnant women were omitted from all analyses of body composition.
Statistical analyses
Age-specific protein intake and standardised BMI, lean mass and fat mass at age 22 years
To fulfil the first aim of this study we assessed the association of age-specific protein intakes relative to needs with three measures of body size at 22 years using multivariable linear regression analyses. Protein intakes relative to needs at ages 2, 11, 15, 19 and 22 years were simultaneously included in the regression models as independent variables. The protein intake variables were not significantly correlated with each other (variance inflation factors <10). To aid in the interpretation of estimates, we reported changes associated with 20 % increases in protein relative to needs – a modest increase that is within the range of realistic dietary changes. (e.g. a 20 % increase is equivalent to an additional 2 g of protein (60 ml of whole milk) for a 2-year-old who weighs 10 kg or an additional 9 g of protein (265 ml of whole milk) for a 22-year-old who weighs 55 kg.) Product terms between sex and protein intake relative to needs were included to assess sex-specific associations. Analyses were adjusted for the above-detailed anthropometric, dietary and socioeconomic covariates. The three outcomes, standardised BMI, standardised lean mass and standardised fat mass, were dependent variables.
Derivation of protein intake trajectories
To meet the second objective of this study, we investigated whether history of protein intake was differentially associated with later BMI. This involved first classifying individuals with similar protein intake trajectories into groups, and then relating trajectory membership to later BMI.
We used finite mixture models to classify the cohort’s heterogeneous patterns of relative protein intake (g/kg body weight) from 2 to 22 years into latent growth curves or trajectories. This statistical approach assumes that individuals classified within the same trajectory have homogenous protein intake, and that their protein intake patterns vary from those of individuals assigned to other trajectories( Reference Jones and Nagin 28 , Reference Nagin 29 ).
An a priori criterion was established to select the optimal number of trajectories. We considered: (i) parsimony: we would select the simplest number (n) of trajectories needed to describe meaningful patterns in the data, and thus, a five-trajectory solution would be the largest tested, (ii) reasonable subset sizes: we would reduce the possibility of capturing idiosyncratic or anomalous protein intake patterns( Reference Louvet, Gaudreau and Thompson 30 ) by ensuring that trajectories represented at least 3 % of the cohort and (iii) Bayesian information Criteria (BIC). For a given model with n trajectories, we modelled relative protein intake by specifying cubic, quadratic or linear trajectories. If P values exceeded 0·10 for the higher order term then a lower order model was preferred for the n-trajectory model( Reference Jones, Nagin and Roeder 31 , Reference Jones 32 ). A similar process was used to select the optimal orders for a model with n+1 trajectories. Next, the a priori criterion was used to compare models for n–n+1 trajectories and the final model was selected. Trajectories were derived using the traj command in Stata 14( Reference Jones and Nagin 28 ).
One-way ANOVA and χ 2 tests were employed to assess whether the distribution of covariate characteristics in early life varied significantly by trajectory membership.
Trajectory of relative protein intake and standardised BMI, lean mass and fat mass at age 22 years
Multivariable linear regression models were then used to estimate the association of the independent variable, protein intake trajectory membership, with the three outcomes (standardised BMI, lean mass and fat mass at age 22 years). Product terms between sex and protein intake trajectories were included to elucidate sex-specific associations. Regression models also included the above-described anthropometric, dietary and socioeconomic factors from early life and adulthood. We used post-estimation Wald tests to compare outcomes between trajectories for males and females separately. (e.g. we assessed whether the difference between the BMI of females aged 22 years assigned to a given trajectory was significantly different from that of females assigned to a referent trajectory.)
We also assessed the potential for bias due to attrition by conducting Heckman( Reference Heckman 33 ) selection models. These two-stage regression models involve (i) using a set of baseline covariates (maternal, household and community-level factors) as independent variables that relate to the outcome of being present in the survey at age 22 years and (ii) using the independent variables (protein intake variables, interaction terms and confounders) to predict the body size outcome (such as BMI). The model compares residuals from both the selection model and the prediction model, and if residuals are uncorrelated then the potential for selection bias in the standard regression coefficients may be mitigated. We compared coefficients from the Heckman selection models with the standard regression models.
Results
Descriptive demographic and dietary details in the Cebu Longitudinal Health and Nutrition Survey
On average, mothers in the CLHNS had received approximately 8 years of formal education at the time of the offspring’s birth (Table 1). The sample had a relatively low urbanicity score in early life but, because of migration to more urban areas as well as urbanisation of all communities, the sample became more urban by age 22 years. By age 22 years, offspring had spent an average of 11 years in formal education and had a mean BMI that was in the normal-weight category. Mean protein intake exceeded the RDA at every survey. Across all surveys, meat and poultry, seafood and grains accounted for >75 % of all protein consumed (data not shown). All other protein sources (such as eggs, dairy products, legumes and seeds) contributed <10 % to protein intake.
Table 1 Select demographic and dietary characteristics from birth to 22 years Cebu Longitudinal Health and Nutrition Survey (1983–2005) (Mean values and standard deviations)

* ANOVA shows that means vary significantly across the survey years shown (P<0·05).
Age-specific intakes of protein were differentially associated with body composition at age 22 years
Protein intake was differentially associated with body composition in an age-dependent manner (Fig. 1). A 20 % higher intake of protein (relative to age-specific RDA (g/kg body weight)) consumed at age 2 years was modestly but positively associated with females’ BMI (0·020 sd; 95 % CI 0·006, 0·033; β equivalent to 0·06 kg/m2) and females’ lean mass (0·014 sd; 95 % CI 0·001, 0·028 or β 0·06 kg) at 22 years.

Fig. 1 Protein intakes relative to needs were differentially associated with fat mass, lean mass and BMI at age 22 years in an age-dependent manner in the Cebu Longitudinal Health and Nutrition Survey (CLHNS). Bars represent the change in early adulthood standardised BMI, fat mass or lean mass, associated with a 20 % increase in protein intake at the indicated age relative to the recommended daily allowance for that age. BMI, fat mass and lean mass at age 22 years were standardised to the mean of the sex-stratified sample of the CLHNS. Coefficients were derived from the linear regression of age-specific protein intakes relative to needs on the standardised outcomes and adjusted for characteristics at birth (offspring weight, maternal education and maternal height), characteristics at age 2 years (offspring BMI and household assets), characteristics from age 22 years (offspring education and assets, physical activity level, carbohydrate residuals, fat residuals and energy intake). ![]() , Males;
, Males; ![]() , females. *P<0·05.
, females. *P<0·05.
At all other ages, protein intake relative to needs was mostly inversely associated with body composition at age 22 years; the strength and magnitude of these associations varied with age. The largest of these estimated associations were for protein consumed at age 22 years. A 20 % higher intake of protein relative to needs was associated with lower lean mass (−0·147 sd; 95 % CI −0·165, −0·129 or β −0·3 kg in females and −0·175 sd; 95 % CI −0·197, −0·153 or β −0·51 kg in males), fat mass (−0·155 sd; 95 % CI −0·175, −0·136 or β −0·4 kg in females and −0·161 sd; 95 % CI −0·184, −0·137 or β −0·4 kg in males) and BMI at 22 years (−0·164 sd; 95 % CI −0·183, −0·145 or β −0·3 kg/m2 and −0·177 sd; 95 % CI −0·201, −0·153 or β −0·3 kg/m2 in females and males, respectively). The Heckman-corrected findings were similar to these results.
Derivation of protein intake trajectories using latent class growth curve analyses
The four-trajectory model was selected because it had optimal parsimony, interpretability and fit statistics (BIC=−12 006·93). Spaghetti plots from random subsets of each trajectory are displayed (Fig. 2(a) and (d)). All latent trajectories of mean protein intake for the 2586 individuals in the CLHNS are shown in Fig. 2(f). The referent trajectory (58 % of the sample) was characterised by ‘normal consumers’ whose protein intake was just slightly higher than recommended (Fig. 2(a)). The second trajectory (20 %) was characterised by ‘high consumers during infancy’ who tended to exceed the RDA at age 2 years but had relatively normal intakes thereafter (Fig. 2(b)). The ‘usually high consumers’ (18 %) tended to exceed the RDA before age 19 years, and had markedly high intakes at age 11 years (Fig. 2(c)). The smallest trajectory (5 %) was characterised by ‘always high consumers’ who had very high protein intakes at age 2 years and high protein intakes thereafter (Fig. 2(d)). All mean intakes for derived trajectories exceeded the RDA (Fig. 2(e)). Although trends were similar for trajectories of absolute protein intake (with normal consumers generally consuming the lowest grams of protein in each survey), the distinctions were not as marked (Fig. 2(f)).

Fig. 2 Trajectories of protein intakes relative to needs from 2 to 22 years in the Cebu Longitudinal Health and Nutrition Survey (n 2586). Fig. 1(a)–(d) each show randomly selected spaghetti plots or trajectories of protein intake relative to needs from actual individuals in the Cebu Longitudinal Health and Nutrition Survey. Four trajectories were derived using latent class growth curve analyses. (a) Subset of the ‘normal consumers’ who constituted 58 % of the sample; (b) ‘high consumers during infancy’ (20 %); (c) ‘usually high consumers’ (18 %); (d) ‘always high consumers’ (5 %); (e) mean protein intake relative to needs (g/kg body weight) by trajectory with 95 % CI; (f) mean absolute protein intakes (g) and standard deviations for those trajectories. ![]() , Usually high consumers;
, Usually high consumers; ![]() , always high consumers;
, always high consumers; ![]() , high consumers during infancy;
, high consumers during infancy; ![]() , normal consumers.
, normal consumers.
Early life characteristics vary by trajectory
Early life characteristics varied significantly by trajectory (Table 2). ‘Normal consumers’ lived in households with fewer assets at age 2 years, lived in more rural communities and had the longest average duration of breast-feeding. Maternal education at baseline was highest for the groups of ‘high consumers during infancy’ and ‘always high’ consumers. The ‘usually high’ consumers had a disproportionately more males whereas members of ‘always high’ consumers had the lowest mean birth weight and BMI at age 2 years.
Table 2 Mean demographic characteristics overall and by trajectory classification
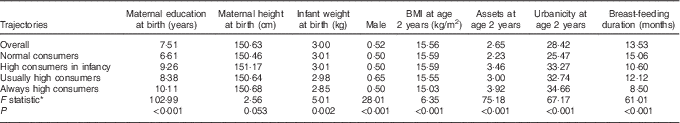
* ANOVA for continuous variables or χ 2 test for categorical variables (sex).
Trajectories of protein intake relative to needs are differentially associated with BMI at age 22 years
Trajectory membership was differentially associated with body composition at age 22 years (Fig. 3). Female or male normal consumers served as the referent category in the sex-specific contrasts. ‘Usually high’ consumption was associated with lower BMI (−0·244 sd; 95 % CI −0·406, −0·083; β equivalent to −1·1 kg/m2 and −0·338 sd; 95 % CI −0·553, −0·122 or β −0·8 kg/m2 in males and females, respectively). ‘Usually high’ consumption was also associated with lower fat mass (−0·170 sd; 95 % CI −0·329, −0·011 or β −1·7 kg and −0·374 sd; 95 % CI −0·594, −0·155 or β −0·8 kg) and lower lean mass (−0·388 sd; 95 % CI −0·541, −0·236 or β −1·7 kg and −0·296 sd; 95 % CI −0·506, −0·086 or β −2·2 kg in males and females, respectively). ‘Always high’ consumption was associated with lower lean mass in males (−0·441 sd; 95 % CI −0·781, −0·101 or β −2·6 kg). In both sexes, high consumers during infancy were similar to normal consumers when BMI, lean mass and fat mass at age 22 years were compared. Similar inferences were drawn when the analytical sample was restricted to participants with data from at least three of five protein survey rounds. Inferences from the Heckman-corrected findings were similar to these results, but a significant association did emerge between high protein intake in infancy and both fat mass and BMI in females.

Fig. 3 Trajectories of protein intake are differentially associated with BMI, lean mass and fat mass at age 22 years in the Cebu Longitudinal Health and Nutrition Survey (CLHNS). Coefficients are derived from the regression of trajectories of protein intake from age 2 to 22 years on standardised BMI, fat mass and lean mass, adjusted for characteristics at birth (offspring weight, maternal education and maternal height), characteristics at age 2 years (offspring BMI and household assets), characteristics from age 22 years (offspring education and assets, physical activity level, carbohydrate residuals, fat residuals and energy intake). BMI, fat mass and lean mass at age 22 years were standardised to the mean of the sex-stratified sample of the CLHNS. Standardised outcomes for one sex in a given trajectory were compared to outcomes for the normal consumer trajectory for that sex. ![]() , Males;
, Males; ![]() , females. *P<0.05.
, females. *P<0.05.
Discussion
In this study, protein intake from age 2 to 22 years had nuanced associations with body composition at age 22 years. Early-childhood protein intakes were positively associated with later BMI and lean mass in females. However, protein intake from age 11 to 22 years tended to be inversely associated with later BMI, lean mass and fat mass. Of the four latent trajectories identified, trajectories marked by high adolescent and adult intakes of protein were associated with lower BMI, lean mass and fat mass at age 22 years.
A growing body of literature has associated infant or early-childhood protein intake with risk of obesity in later childhood or even adulthood( Reference Michaelsen, Larnkjær and Mølgaard 5 , Reference Koletzko, Broekaert and Demmelmair 6 , Reference Hörnell, Lagström and Lande 9 , Reference Koletzko, Dobrzanska and Sengier 34 – Reference Pimpin, Jebb and Johnson 36 ). For example, protein intake (g/kJ (g/kcal)) from 9 to 12 months was associated with higher BMI in 6-year-old Icelandic males( Reference Gunnarsdottir and Thorsdottir 37 ) whereas high protein intake (g/kJ (g/kcal)) in 12-month-old German males was associated with higher BMI at age 7 years( Reference Günther, Remer and Kroke 38 ). Our current findings lend credence to the early protein–later BMI hypothesis.
However, our analyses differed from previously published studies in several ways. First, published studies conducted associations with BMI and did not include measures of lean or fat mass as we did here; it is unclear whether their associations with BMI also extended to adiposity or lean mass. Our results indicate that early protein was associated with higher female BMI and lean mass, but not fat mass. In this low-income cohort, this could also be interpreted as females who were better nourished in early life being less stunted and underweight as adults. Infant protein intake also programmes linear growth, an association potentially mediated by the insulin-like growth factor( Reference Hoppe, Udam and Lauritzen 39 , Reference Putet, Labaune and Mace 40 ). A recent study in this Cebu cohort did find that childhood protein intakes (g) were positively associated with attained height at age 22 years( Reference Bhargava 41 ). As protein is associated with both lean and fat mass, it is important to explicitly investigate whether associations with BMI are due to lean mass, fat mass or both, as we did here.
Second, unlike other cohorts in which similar analyses were conducted, this Filipino cohort had high prevalence of infant undernutrition and the infant diet tended to be poor in quality (low in animal protein and rich in energy from grain-based porridges)( Reference Wright, Bentley and Mendez 42 ). Differences in protein source and/or quality presumably drive the associations of protein intake with height( Reference Hoppe, Udam and Lauritzen 39 ) and body size( Reference Günther, Remer and Kroke 38 ). We recently showed that even within CLHNS, associations between protein intake and concurrent BMI vary for protein from animal and plant sources( Reference Wright, Mendez and Sotres-Alvarez 43 ). The dietary differences in our cohort may have contributed to the null associations observed for early protein intake with later BMI, lean mass and fat mass in males, and fat mass in females.
We also found that protein intakes (from ages 11 to 22 years) were strongly and inversely associated with lean mass, fat mass and BMI in both sexes. A study in a German cohort reported positive associations of animal protein intake at around the time of the adiposity rebound with men’s adulthood fat-free mass( Reference Assmann, Joslowski and Buyken 44 ). During infancy, BMI tends to increase during the first year of life and then declines until age 3–5 years, after which it ‘rebounds’ – the adiposity rebound is this increase in BMI following the minimal point or nadir seen in the BMI growth charts of children. As the CLHNS was not conducted during this period, we were unable to directly compare results with this study. Nevertheless, in this same German cohort, protein intake after infancy tended to be positively associated with later lean mass: tertiles of pubertal (between 9 and 15 years) energy-adjusted animal protein intake were associated with higher women’s fat-free mass at 18–25 years( Reference Assmann, Joslowski and Buyken 44 ). These findings contradict ours. Differences in diet quality may again help to explain why our findings vary. In addition, we expressed protein in g/kg body weight and this specification may also explain why the results were not fully concordant with earlier studies that expressed protein intake in absolute grams or percentiles. Future studies should clarify how protein relative to needs, absolute protein intake as well as protein quality relates to later adulthood body size.
Our trajectory analyses also suggest that adolescent and adult intakes drove differences in body composition: trajectories characterised by higher protein intakes at age 22 years were consistently associated with lower BMI, fat mass and lean mass but body composition of the high infant trajectory was similar to the normal-consumption category. The estimated age-specific association of the early childhood diet with adult body size was relatively much smaller in magnitude compared with later years. Indeed, the Heckman-weighted, inverse associations that emerged between the trajectory of high protein intake in infancy and fat mass and BMI in females conflict the early protein hypothesis. Future studies should account for diet throughout the life course, so the relative contribution of protein intake at different ages to later body size is not missed.
These findings must be contextualised within the greater limitations of this study. First, as this study was conducted in an observational cohort, the findings presented here are associational and are not causal. We attempted to control for factors that were related to the protein intake variables and body size outcomes, but it is possible that omitted factors (such as the quality or source of protein) or poorly measured confounders may leave our estimates residually confounded. Second, in this rapidly urbanising society, the study sample decreased from 3080 at birth, to 2456 individuals at 24 months and 1885 participants at 22 years( Reference Drewnowski and Popkin 14 ). We found that Heckman-corrected findings were generally similar to the results reported here. Nevertheless, there is still potential for selection bias if participants and those who were lost to follow-up varied based on unmeasured factors.
The third limitation is that we were unable to include dietary data from birth to 2 years. The inclusion of infant intakes would have facilitated comparisons with other DOHaD studies of the early protein hypothesis. As breastmilk nutrients were not quantified, the use of infant nutrient data would have underestimated total protein intake. Dietary data from infancy and at the time of the adiposity rebound may have improved trajectory derivation and strengthened these life-course analyses. Finally, the latent class trajectories derived here are driven by the unique socioeconomic and dietary characteristics of this population. Whereas the insights are valuable, they may not be generalisable to other populations.
Despite its limitations, this is an important methodological contribution to the life-course epidemiology literature. Ours is one of few studies to classify longitudinal dietary exposures and relate them to later health. Only one other study has analysed trajectories of dietary protein; in this German cohort, researchers classified individuals into trajectories of below/above the median protein intakes at ages 12 and 18–24 months. They found that high protein intake at both time-points was associated with significantly greater standardised BMI and percentage body fat at age 7 years( Reference Jones and Nagin 28 ). Although this study captured the early life exposures that we were unable to include here, the crude derivation of trajectories, short follow-up period and childhood outcome clearly limit its generalisability. To our knowledge, only one other study has analysed a macronutrient across such a wide breadth of the life course: researchers used cluster analysis to isolate fat-intake patterns from 2 to 18 years in a German cohort( Reference Alexy, Schultze-Pawlitschko and Sichert-Hellert 45 ); they later found that these patterns did not explain later BMI( Reference Alexy, Sichert-Hellert and Kersting 46 ). Another study of Chinese adults used latent class trajectory analysis to group individuals according to a dietary-pattern score, they found that those with healthier dietary patterns had lower HbA1c, and when trajectories with recent healthy dietary patterns were compared, those who had prolonged recent exposure to a healthy dietary pattern had lower HbA1c compared with those who whose trajectory showed recent improvements in dietary pattern( Reference Batis, Mendez and Sotres-Alvarez 47 ). These longitudinal perspectives grant insights into the potentially cumulative role of diet in later health. The novelty of our study is that we were able to demonstrate how both varied patterns of protein intake and period-specific exposures were associated with later body composition within a single cohort.
Overall, our study supports long-term associations of protein intake with late BMI. However, more research is needed to clarify whether these associations are also present in other socioeconomic contexts. Analysis of comprehensive dietary data from multiple time-points and longer follow-up periods would further identify optimal ranges of age-specific protein intake for promoting later health and disease.
Acknowledgements
The authors express gratitude for the assistance of Dr Barry Popkin and Dr Allison Aiello for their insights throughout this project.
This study was funded by the Howard Hughes Medical Institute International Student Research Fellowship.
M. W. formulated the research questions, designed the study, analysed the data and wrote the article; L. A. provided the databases necessary; M. A. M., D. S.-A. and L. A. provided guidance in the analysis of data, the interpretation of findings and the critical revision of the manuscript; L. A. was responsible for the final content. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.










