Inflammation is an important factor contributing to cancer( Reference Touvier, Fezeu and Ahluwalia 1 , Reference Keibel, Singh and Sharma 2 ), and considerable evidence for the role of chronic inflammation in prostate cancer is accumulating( Reference Kopp, Friis and Christensen 3 – Reference Nakai and Nonomura 5 ). While inflammation typically occurs as part of the body response to tissue insult/injury( Reference Keibel, Singh and Sharma 2 , Reference Pan, Lai and Dushenkov 6 ), chronic inflammation is a persistent condition in which tissue destruction and repair occur simultaneously( Reference Coussens and Werb 7 , Reference Philip, Rowley and Schreiber 8 ). This involves the continuous recruitment of pro-inflammatory cytokines associated with increased blood flow to the injured tissue, due to histamine released by damaged mast cells( Reference Keibel, Singh and Sharma 2 ).
A recent case–control study has shown that the levels of C-reactive protein are higher in men with prostate cancer than in those with benign prostatic hypertrophy( Reference Kim, Jeon and Lee 9 ), and the Melbourne Collaborative Cohort Study has reported higher levels of IL-6, a pro-inflammatory cytokine, among malignant prostate cancer cases compared with those with benign prostate disease( Reference Tindall, Severi and Hoang 10 ). These data are consistent with the hypothesis that innate immunity and inflammation play a role in prostate cancer( Reference Kazma, Mefford and Cheng 11 ).
Diet represents a complicated set of exposures that often interact and whose cumulative effect modifies both inflammatory responses and health outcomes. Although several studies have been conducted, the relationship between diet and prostate cancer is still unclear( Reference Kolonel 12 , Reference Gronberg 13 ). According to the Second Expert Report from the World Cancer Research Fund( 14 ), foods containing lycopene and Se are protective against prostate cancer, while diets high in Ca increase its risk. A positive association between elevated intake of meat( Reference Kolonel 12 , Reference Giovannucci, Rimm and Colditz 15 , Reference Talamini, Franceschi and La Vecchia 16 ) and milk and dairy products( Reference La Vecchia, Negri and D'Avanzo 17 – Reference Le Marchand, Kolonel and Wilkens 20 ) with the risk of prostate cancer has been observed. Conversely, an inverse association has been found for vegetable intake, but the results have been inconsistent( Reference Giovannucci, Ascherio and Rimm 21 – Reference Hsing, McLaughlin and Schuman 24 ). The possible relationship between inflammation derived from dietary exposure and the risk of prostate cancer has not been investigated.
The paucity of research related to diet and inflammation is probably due to logistic issues resulting from methodological complexity involved in linking diet, inflammation and cancers in the same study. In an effort to fill this methodological gap, researchers at the University of South Carolina's Cancer Prevention and Control Program developed a dietary inflammatory index (DII), which can be used in diverse populations in order to predict the levels of inflammatory markers and related health outcomes( Reference Shivappa, Steck and Hurley 25 , Reference Shivappa, Steck and Hurley 26 ). The development of the DII involved careful review and scoring of the scientific literature on diet and inflammation, and obtaining of datasets from around the world for comparison with dietary intakes of individuals( Reference Shivappa, Steck and Hurley 25 ). Thus far, the DII has been found to be associated with inflammatory cytokines including C-reactive protein and IL-6( Reference Shivappa, Steck and Hurley 26 – Reference Wood, Shivappa and Berthon 28 ), the glucose-intolerance component of the metabolic syndrome, increased odds of asthma and reduced forced expiratory volume in 1 min (FEV1), shift work and colorectal cancer (CRC) among women from the Iowa Women's Health Study( Reference Wirth, Burch and Shivappa 27 – Reference Shivappa, Prizment and Blair 29 ).
The purpose of the present study was to examine the association between the DII and the risk of prostate cancer in a case–control study conducted in Italy. A previous case–control study has revealed a positive association with the increased intake of milk and dairy products and a possible protective effect of vegetable intake( Reference Bosetti, Micelotta and Dal Maso 30 ).
Methods
Full details of the case–control study have been published elsewhere( Reference Bosetti, Micelotta and Dal Maso 30 ). Briefly, the study was conducted between 1991 and 2002 in four Italian areas, including the greater Milan area and the provinces of Pordenone and Gorizia in northern Italy, the province of Latina in central Italy, and the urban area of Naples in southern Italy. Cases were 1294 patients aged < 75 years (median age 66 years, range 46–74 years) who were admitted to major teaching and general hospitals in the areas under study with incident, histologically confirmed carcinoma of the prostate, diagnosed no longer than 1 year before the interview. Controls were 1451 subjects aged < 75 years (median age 63 years, range 46–74 years) who were selected among patients admitted to the same hospitals as cases for a wide spectrum of acute, non-neoplastic conditions, not related to known or potential risk factors for prostate cancer and long-term modifications of diet. The main diagnostic categories of controls were traumatic conditions, mostly sprains and fractures (21 %); non-traumatic orthopaedic disorders, such as low back and disc disorders (33 %); acute surgical conditions, mostly abdominal such as appendicitis or strangulated hernia (17 %); and other illnesses, such as eye, ear, nose, skin and dental disorders (29 %).
Information on sociodemographic characteristics, anthropometric measures, lifestyle habits, including smoking status and alcohol drinking, personal medical history, and family history of cancer in first-degree relatives was assessed during subjects' hospital stay using a standard questionnaire that was administered by trained interviewers to the cases and controls. The subjects' usual diet during the 2 years before the diagnosis of cancer (or hospital admission, for controls) was assessed using an interviewer-administered FFQ, including seventy-eight foods and beverages, as well as a range of the most common Italian recipes. Subjects were asked to indicate the average weekly frequency of consumption of each dietary item; intakes lower than once per week, but at least once per month, were coded as 0·5 per week. Nutrient and total energy intake was determined using the Italian food composition database( Reference Gnagnarella, Parpinel and Salvini 31 ). The FFQ showed satisfactory validity( Reference Decarli, Franceschi and Ferraroni 32 ) and reproducibility( Reference Franceschi, Negri and Salvini 33 , Reference Franceschi, Barbone and Negri 34 ) with Spearman's correlation coefficients between 0·50 and 0·60 for validity and between 0·60 and 0·70 for reproducibility.
Briefly, to calculate the DII for the subjects of the present study, the dietary data were first linked to the world database that provided a robust estimate of the mean and standard deviation for each food parameter considered( Reference Shivappa, Steck and Hurley 25 ). This was achieved by subtracting the ‘standard global mean’ from the intake reported via the FFQ and dividing this value by the standard deviation (both calculated from the world database) in order to get z scores. To minimise the effect of ‘right skewing’, these z scores were then converted to a centred percentile score. The centred percentile score of each food parameter for each subject was then multiplied by the respective effect score of food parameters (inflammatory potential for each food parameter), which was derived from the literature review, in order to obtain a food parameter-specific DII score for a subject. All of the food parameter-specific DII scores were then summed to create the overall DII score for each subject in the study( Reference Shivappa, Steck and Hurley 25 ). A description of the validation work, including both dietary recalls and a structured questionnaire similar to a FFQ, is also available( Reference Shivappa, Steck and Hurley 26 ).
The food parameters used for the calculation of the DII were carbohydrate, protein, fat, alcohol, fibre, cholesterol, saturated fat, monounsaturated fat, polyunsaturated fat, n-3 PUFA, n-6 PUFA, niacin, thiamin, riboflavin, vitamin B6, Fe, Zn, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, β-carotene, anthocyanidins, flavan-3-ol, flavonol, flavonones, flavones, isoflavones, caffeine and tea. A higher DII score indicates a more pro-inflammatory diet and a lower DII score indicates a more anti-inflammatory diet. BMI was calculated as weight (in kg) divided by height (in m) squared, and was categorised into < 24·0, 24–25·9, 26·0–28·9 and ≥ 29·0 kg/m2 ( Reference Danesh, Whincup and Walker 35 ).
Statistical analyses
The DII was analysed both as a continuous variable and by quartiles of exposure. The DII (as quartiles) was examined using the ANOVA test for continuous variables or the χ2 test for categorical ones across the following characteristics: age; years of education; BMI; smoking status; family history of prostate cancer. To understand the dietary profile of each quartile of the DII, we examined the distribution of various food groups across the quartiles of the DII. An ANOVA was used to test for the differences among the dietary groups. We estimated the OR and the corresponding 95 % CI using logistic regression models, adjusted for age, study centre, years of education, social class, BMI, smoking status, family history of prostate cancer, and total energy intake( Reference Breslow and Day 36 ). The covariates were chosen a priori as they have been previously shown to be the risk factors of prostate cancer. Tests for linear trend were performed using the median value of each quartile as an ordinal variable. Statistical tests were performed using SAS® 9.3 (SAS Institute, Inc.). All P values were two-sided.
Results
The distribution of prostate cancer cases and controls according to age, years of education, and other selected variables is presented in Table 1. The cases were somewhat older than the controls (>50 v. ≤ 50 years: OR 2·3, 95 % CI 1·3, 4·3), were more highly educated ( ≥ 12 years of education v. < 7 years of education: OR 1·9, 95 % CI 1·5, 2·3), had a higher social class (lower v. higher: OR 2·4, 95 % CI 1·7, 3·0), and more often reported a first-degree relative with prostate cancer (family history of prostate cancer v. no family history OR 3·9, 95 % CI 2·5, 5·8). No differences in the BMI level were observed between cases and controls. All factors were included in the analysis.
Table 1 Distribution of 1294 cases of prostate cancer and 1451 controls according to age, years of education, and other selected covariates in the study conducted in Italy between 1991 and 2002 (Number of subjects and percentages)
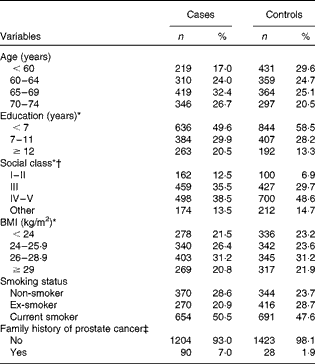
* The sum does not add up to the total because of some missing values.
† I–II: professional, managerial, intermediate; III: skilled occupations; IV–V: partly skilled and unskilled occupations; other: farmers, and other or unknown occupations.
‡ In first-degree relatives.
Small differences were observed in sociodemographic characteristics, anthropometric measures and lifestyle habits across the quartiles of the DII (data not shown). However, current smokers were more frequently in the higher quartiles of the DII than in the lower quartile, i.e. 32 % of those in the highest quartile v. 26 % of the controls in the lowest quartile. There were fewer men who were overweight or with a family history of prostate cancer in the higher quartiles of the DII.
Concerning the distribution of various food groups, there was a significant reduction in the consumption of vegetables, fruits, poultry and fish, and a significant increase in the consumption of pork, sugars, cheese and bread across the quartiles of the DII (Table 2). The OR and corresponding 95 % CI of prostate cancer according to the quartiles of the DII are shown in Table 3. In the age-adjusted models, no meaningful association was found between the DII and the risk of prostate cancer. However, in multivariable analysis, significant positive associations were found, with an OR of 1·06 (95 % CI 1·00, 1·13) for a one-unit increment in the DII (corresponding to approximately 7 % of its global range)( Reference Shivappa, Steck and Hurley 25 ). In the analysis using the DII expressed as quartiles, a significant trend of increasing risk (P trend= 0·04) was found; however, there was some indication of flattening in the last two quartiles (ORQuartile 3v. 1 1·32, 95 % CI 1·03, 1·69 and ORQuartile 4v. 1 1·33, 95 % CI 1·01, 1·76) when compared with men in the lowest quartile of the DII.
Table 2 Distribution of food groups across quartiles of the dietary inflammatory index (DII) in the study conducted in Italy between 1991 and 2002 (Mean values and standard deviations)
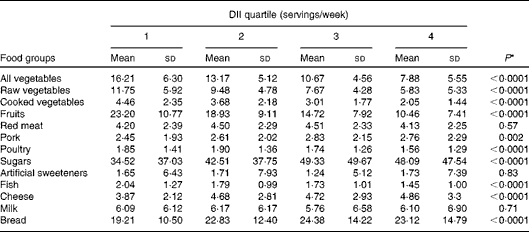
* P values were obtained from ANOVA.
Table 3 Odds ratios of prostate cancer according to quartiles of the dietary inflammatory index (DII), among 1294 cases and 1451 controls, in the study conducted in Italy between 1991 and 2002 (Odds ratios and 95 % confidence intervals)
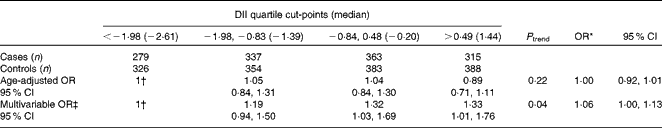
* Continuous OR for a one-unit increment in the DII, corresponding to approximately 7 % of its global range.
† Reference category.
‡ Adjusted for age, study centre, BMI, years of education, social class, smoking status, family history of prostate cancer, and total energy intake.
Discussion
The present study, being one of the largest case–control investigations on diet and prostate cancer to date in a southern European population, shows a positive association between the DII and the risk of prostate cancer with statistically significant risk estimates for the DII expressed as a continuous variable and for men in the third and fourth quartiles of the DII (v. the first quartile). However, there was a levelling of risk across the two highest quartiles. We also observed a reduction in the consumption of healthy food items such as vegetables, fruits and fish, and an increase in the consumption of unhealthy food items such as pork, cheese and sugars, with increasing DII scores. This result supports the hypothesis that men who consume a pro-inflammatory diet are at a higher risk of developing prostate cancer( Reference Kazma, Mefford and Cheng 11 ).
Various dietary factors exert an array of effects on prostate cancer; some of these are pro-inflammatory (e.g. meat intake( Reference Cross, Peters and Kirsh 4 )) and some are anti-inflammatory (e.g. isoflavone( Reference Travis, Spencer and Allen 37 ) and soya( Reference Hebert, Hurley and Olendzki 38 ) intake). The positive association of the DII with the risk of prostate cancer found in the present case–control study is of specific interest. One of the possible mechanisms responsible for this association is the effect of the pro-inflammatory diet on systemic inflammation and insulin resistance( Reference Esmaillzadeh, Kimiagar and Mehrabi 39 , Reference Festa, D'Agostino and Howard 40 ). Consumption of foods such as meat and butter have been shown to increase systemic inflammation by increasing levels of high-sensitive C-reactive protein, E-selection and soluble vascular cell adhesion molecule-1( Reference Esmaillzadeh, Kimiagar and Mehrabi 39 ), which then are responsible for increasing insulin resistance( Reference Festa, D'Agostino and Howard 40 ). Increasing insulin resistance leads to hyperinsulinaemia, which has been demonstrated to play a role in the development of prostate cancer by inhibiting apoptosis, stimulating cell proliferation( Reference Kaaks and Lukanova 41 ) and influencing the insulin-like growth factor axis with consequent alterations in sex hormone metabolism. Along this line, a diet high in glycaemic load has been related to an increased risk of prostate cancer( Reference Augustin, Galeone and Dal Maso 42 ). Also, a diet rich in pro-inflammatory constituents, such as saturated fat, causes proliferation, inflammation and oxidative stress that can lead to benign prostatic hyperplasia, prostatitis, and possibly cancer of the prostate( Reference Vykhovanets, Shankar and Vykhovanets 43 ).
The influence of diet on prostate cancer is difficult to evaluate, and challenges in dietary exposure assessment are greatest in case–control studies. A human diet consists of both pro-inflammatory and anti-inflammatory foods, nutrients and other food constituents; thus, the DII score, which takes into account both categories of dietary exposure, more accurately reflects the relationship of diet with the risk of cancer than individual nutrients. Although (hospital-based) case–control studies are thought to be more susceptible to selection and information bias than cohort studies( Reference Breslow and Day 36 ), several factors argue in support of the validity of the present investigation. Dietary information was elicited using a valid and reproducible FFQ( Reference Decarli, Franceschi and Ferraroni 32 – Reference Franceschi, Barbone and Negri 34 ), which was comprehensive enough to allow adjustment for total energy intake. This was administered to cases and controls by the same interviewers under similar conditions. To minimise any recall bias due to the onset or treatment of the disease, individuals were asked about food intake in the 2 years before the interview. The potential bias in the recall of food intake, however, should be limited in Italy, because the diet and prostate cancer issue has not had widespread interest. Also, controls were selected from the same hospital system as the cases and therefore are more likely to be subject to similar sorts of recall bias. Indeed, hospital-derived controls should be less prone to information bias than population ones( Reference Breslow and Day 36 ). Furthermore, the cases and controls were selected from the same catchment areas, the participation rate was high, particular attention was paid to exclude from the control group diseases potentially linked to the diet and long-term dietary modifications, and major confounding factors of prostate cancer were accounted for.
It also should be made clear that not all of the forty-five food parameters mentioned in the study on the development of the DII( Reference Shivappa, Steck and Hurley 25 ) were used for the calculation of the DII. For the current DII calculation, we used the data from thirty-two food parameters, and most of these had article weights of >236 (indicating optimal confidence in the evidence base). However, we do understand that about half of the food parameters had article weights below the median level of 236. Many of these food parameters were not studied extensively in the current scenario. These include foods such as rosemary, thyme and oregano that are consumed in relatively small amounts. Hence, we feel that there is no immediate need to update the literature review; however, we may consider updating in the future.
The regionally representative database was created to include dietary consumption of the forty-five food parameters from eleven countries. These eleven countries were selected from different regions of the world, in order to obtain a wide spectrum of consumption of these food parameters. The countries included are the USA, Mexico, England, Denmark, India, Australia, New Zealand, Bahrain, Scotland, South Korea and Japan( Reference Shivappa, Steck and Hurley 25 ). So, the mean values of the food parameters from this database should be representative of the average consumption of these parameters across the world. Notwithstanding the limitations of case–control studies in general, we believe that our findings of a positive association of the DII with the risk of prostate cancer are plausible and could be related to immune and hormonal factors( Reference Kaaks and Lukanova 41 , Reference Vykhovanets, Shankar and Vykhovanets 43 , Reference Pandey and Gupta 44 ).
In conclusion, this uniquely large study on prostate cancer and the DII conducted in a southern European population indicates a possible role of diet in prostate cancer risk through the process of inflammation. However, confirmatory results from other studies conducted in different populations with different study designs are required to truly establish this association.
Acknowledgements
The present study was supported by the Italian Foundation for Cancer Research (FIRC) and the IRCCS Centro di Riferimento Oncologico, Aviano (Ricerca Corrente 2009), Italy. J. R. H. was supported by an Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the National Cancer Institute (K05 CA136975).
The authors' contributions are as follows: N. S. computed the dietary inflammatory index, contributed to the design of the analyses, led statistical analyses, interpreted results and wrote initial drafts of the manuscript; C. B. helped with the design and implementation of the study, data management, consulted on statistical analyses, interpretation of results of statistical analyses, and writing and revising of the manuscript; A. Z., M. M., D. S. and C. L. V. contributed to the study design, data collection, data interpretation, and revision of the manuscript; J. R. H. invented the dietary inflammatory index, helped to design statistical analyses, interpreting results of statistical analyses, and writing of the manuscript.
The authors declare that there are no conflicts of interest.








