1 Introduction
Previous work on Folgefonna (Reference Davies, Vincent and BrimblecombeDavies and others 1982) has shown that not only are the majority of ions removed from the overlying snow and firn early in the melt sequence (fractionation), but that some ionic species are removed preferentially (preferential elution). This suggests that this latter process could result in the early removal of hydrogen ions (relative to other cations), together with ![]() and
and ![]() ions, as strong acids. However the results from the previous study are difficult to interpret as they come from a 60 m core which, in the upper 20 m at least, represents a combination of ice from which the majority of ions would have been removed during a previous melt season and the meltwater percolating downwards from the snow melting near the surface. The experiments described here were designed to look at the processes of preferential elution and fractionation under more controlled conditions, both in the field and in the laboratory.
ions, as strong acids. However the results from the previous study are difficult to interpret as they come from a 60 m core which, in the upper 20 m at least, represents a combination of ice from which the majority of ions would have been removed during a previous melt season and the meltwater percolating downwards from the snow melting near the surface. The experiments described here were designed to look at the processes of preferential elution and fractionation under more controlled conditions, both in the field and in the laboratory.
2 Methodology
2.1 Field experiments
These were performed near the summit of Folgefonna (altitude 1600 m) close to the site used by Reference Davies, Vincent and BrimblecombeDavies and Others (1982) in their coring work during 1981 (Fig.1). The 1983 melt season was particularly late and, compared with 1981, little ablation of the winter snow had occurred before the beginning of these experiments. A pit 2.5 m deep was dug through the surface snow of the ice cap and short horizontal tunnels (referred to as “nests”), 50 cm long and 30 cm square and offset from each other horizontally, were dug into the side of the pit at depths of 0.2, 0.4, 0.5, 0.9 and 1.8 m. The roofs of these tunnels were convexed, and a funnel and clean collecting bottle placed beneath the lowest point of the roof of each to collect meltwater percolating down through the snowpack. Only the results from the nest at the depth of 1.8 m are presented here. During the day on which the nest was constructed (11 July) no meltwater was collected although from 8 to 10 cm of surface ablation was measured during the day. This was thought to be due to the meltwater being carried away along previously established channels. The next morning (12 July), a heat lamp (275 W) was mounted 30 cm above the snow directly over the nest to establish new meltwater channels and after about 1 h (during which time 2 to 3 cm of ablation had occurred under the lamp) meltwater reached the nest below. The lamp was removed and meltwater was collected at the nest for 4 d. Samples were collected hourly during daylight hours while overnight a large container was left to give an integrated sample. Measurements usually commenced at about 10.00 h each morning. pH was measured on site within a few hours of collection using a Corning 113 pH meter and the samples were stored at ~0°C in snow-pits, before being returned to the laboratory where they were stored at 3°C for about 8 weeks before analysis.

Fig.1 Field melt experiments were undertaken on Folgefonna, situated close to the west coast of Norway, at the location indicated.
In addition to the melt samples from the nests, snow cores 2 m long were taken within 5 m of the location of the melt experiment. Three cores (A, B and C) were extracted on 8 July, the first day the expedition was at the melt site, and two more (D and E) were taken on 15 July, as the melt experiment was concluded. These snow cores were sectioned, labelled and bagged, then stored in a deep snow-pit until the conclusion of the expedition when they were lifted by helicopter to a nearby helipad and transferred to a chest freezer at −18°C. They were then returned frozen to the UK and kept in a cold store until immediately before analysis.
Sample handling in the laboratory was carried out under clean conditions, using a laminar flow cabinet and stainless steel or PTFE-coated tools. Melted core samples and meltwater from the nests were filtered through 0.22 μm cellulose acetate filters before analysis. Anions were measured using a Dionex Model 12 ion Chromatograph with detection limits of better than 0.2 μeq 1−1 (~0.01 ppm) for ![]() ,
, ![]() and Cl−. Precision was better than ±3%. Cation concentrations were determined using a Pye Unicam SP9 atomic absorption spectrophotometer with detection limits and precision for Na+, Mg2+, Ca2+, and K+ similar to those of the anions. No ammonium determinations were made.
and Cl−. Precision was better than ±3%. Cation concentrations were determined using a Pye Unicam SP9 atomic absorption spectrophotometer with detection limits and precision for Na+, Mg2+, Ca2+, and K+ similar to those of the anions. No ammonium determinations were made.
The measurement of pH has remained the least satisfactory part of the analytical procedure and, whereas the precision of these pH measurements is to within 0.1 pH units, their accuracy is likely to be very much less. The field measurements of pH involved manual stirring and calibration against strong buffers, whereas recent work by Reference McQuaker, Kluckner and SandbergMcQuaker and others (1983) has indicated that accurate laboratory pH measurements should be made using quiescent samples and that calibration should be against strong acid solutions. They suggest that field determinations of pH are unlikely to be better than 0.1 or 0.2 pH units.
2.2 Laboratory experiments
Snow was collected from a fresh drift (within 24 h of the end of the snowfall) in the Cairngorm mountains, Scotland and returned to a cold store at −4°C. Although the snow drift was apparently uniform and structureless, five samples taken at random from amongst the snow collected showed considerable differences in ionic concentrations (![]() had a mean of 44 μeq 1−1 and a standard deviation of 43 μeq 1−1). For each melt experiment approximately 8 kg of the snow was homogenized with an overhead stirrer using only PTFE-coated tools, until chemical analyses of random samples were the same (within the repeatability of the analysis technique, usually better than 3%).
had a mean of 44 μeq 1−1 and a standard deviation of 43 μeq 1−1). For each melt experiment approximately 8 kg of the snow was homogenized with an overhead stirrer using only PTFE-coated tools, until chemical analyses of random samples were the same (within the repeatability of the analysis technique, usually better than 3%).
The bulk concentration ratio of Na+ and Mg2+ of 3.9:1 (in μeq 1−1) is very close to that of sea-water as are the concentration ratios of Na+, Cl− and ![]() , with little evidence of excess
, with little evidence of excess ![]() due to pollutant sources. The very low
due to pollutant sources. The very low ![]() values are also indicative of a relatively clean air-mass source for this snow.
values are also indicative of a relatively clean air-mass source for this snow.
The experiments were carried out in a simple cylindrical container made from PVC plastic, with a base consisting of a central funnel collector and an outer concentric collection region (Fig.2). The two collecting points for meltwater were used to attempt to differentiate between meltwater which percolated down through the snow and that which either ran down the inside surface of the cylinder or was melted directly by the cylinder (due to the different radiation absorption properties of snow and plastic). The experiments were carried out in a cold room set at −2°C (although air temperatures fluctuated ±2°C on a time scale of a few minutes) with the cylinder wrapped in a closed-cell foam mat to increase the insulation and reduce edge melting effects. Melting was by a 275 W infrared lamp suspended between 20 and 30 cm above the snow surface. Sample treatment and analyses were as for the field samples.

Fig.2 Experimental configuration for the laboratory melt experiments. The inner cylinder has a diameter of 25 cm and a height of 30 cm.
3 Results and Discussion
3.1 Field experiments: snow cores
The cores showed great variability between sites that were only 1 m apart, as was observed by Reference Brimblecombe, Tranter, Abrahams, Blackwood, Davies and VincentBrimblecombe and others (1985). The ion concentrations of the first three cores, A, B and C, collected on 8 July, generally increased with depth but showed considerable variations in concentration (a factor of 2 to 3) over distances of a few centimetres. Table I lists the volume-weighted average concentrations of the major anions and cations in cores A and B and also in the two cores, D and E, which were collected 7 d later. The maximum and minimum concentrations are also given. Figure 3 shows the profiles of ![]() and Na+ in cores A and B, and illustrates the degree of spatial variability in the snow; these are typical of the ion profiles and comparative concentrations found in all the cores. The high concentrations in cores A, B and C are associated with firn layers and ice lenses in the snow (Fig.3); these inhomogeneities in the snowpack channel the downward percolation of meltwater and may also inhibit the flow for a limited time. The two cores collected 7 d later (D and E) show much lower concentrations, particularly in the lower sections, (note 40 cm ablation) and reflect the complete ablation of the top 40 cm of snow and downward percolation of much of this meltwater accompanied by considerable removal of solute from the snowpack. However a simple fractionation mechanism is not sufficient to account for all the ion removal because solar radiation (direct melting) will only penetrate about a decimetre into the snow (Reference O’Neill and GrayO’Neill and Gray 1973). The percolating meltwater must be responsible for removing the ions lower down the cores, either by partial melting due to the temperature of the meltwater being above 0°C or possibly by a chromatographic effect between the snow crystals and the meltwater. In either case, these data strongly suggest that the majority of the solute in the snow is associated with the surface of the snow crystals where it can be easily removed.
and Na+ in cores A and B, and illustrates the degree of spatial variability in the snow; these are typical of the ion profiles and comparative concentrations found in all the cores. The high concentrations in cores A, B and C are associated with firn layers and ice lenses in the snow (Fig.3); these inhomogeneities in the snowpack channel the downward percolation of meltwater and may also inhibit the flow for a limited time. The two cores collected 7 d later (D and E) show much lower concentrations, particularly in the lower sections, (note 40 cm ablation) and reflect the complete ablation of the top 40 cm of snow and downward percolation of much of this meltwater accompanied by considerable removal of solute from the snowpack. However a simple fractionation mechanism is not sufficient to account for all the ion removal because solar radiation (direct melting) will only penetrate about a decimetre into the snow (Reference O’Neill and GrayO’Neill and Gray 1973). The percolating meltwater must be responsible for removing the ions lower down the cores, either by partial melting due to the temperature of the meltwater being above 0°C or possibly by a chromatographic effect between the snow crystals and the meltwater. In either case, these data strongly suggest that the majority of the solute in the snow is associated with the surface of the snow crystals where it can be easily removed.
Table I Average (a), Maximum (b) and Minimum (c) Concentrations in μeq 1−1 of the Major Ions in Four Snow Cores. A and B were collected on 8 July, and D and E on the 15 July. The average depletion of each ion from the cores over this period is also shown and a rank order of preferential elution suggested.)


Fig.3 Ion concentration profiles for ![]() and Na+ in snow cores. Left: spatial variability between two cores, A and B, collected from positions 1 m apart on the same day (8 July), together with the positions of the ice/firn lenses in the cores. Right: temporal changes between core A and core D which was collected 7 d later.
and Na+ in snow cores. Left: spatial variability between two cores, A and B, collected from positions 1 m apart on the same day (8 July), together with the positions of the ice/firn lenses in the cores. Right: temporal changes between core A and core D which was collected 7 d later.
3.2 Field experiments – meltwater from the nest
The time sequences of the concentrations of the four major ions Na+, ![]()
![]() and Cl− plus H+ are shown in Figure 4.
and Cl− plus H+ are shown in Figure 4.

Fig.4 Meltwater concentrations for five major ions at the 1.8 m nest over the 4 days of the melt experiment. Time in hours on each day is shown. H+ is shown on both graphs for comparative purposes but note the change in the ordinate scale.
-
12 July: melting was initiated with the 275 W lamp (10.50–11.40 h) and, although solar radiation was strong resulting in a high ablation rate and a consequent high meltwater flow rate, the ionic concentrations remained almost constant throughout the day (Fig.4), due perhaps to the complete melting of the top 2 or 3 cm of snow by the lamp. The pH was 4.65 and the
 :
:  ratio was 1.7 ± 0.13. Concentrations of Ca2+, Mg2+ and K+ were low, <0.5 μeq 1−1 in total.
ratio was 1.7 ± 0.13. Concentrations of Ca2+, Mg2+ and K+ were low, <0.5 μeq 1−1 in total. -
13 July: a clear, still day with strong insolation.
 and
and  levels peaked at 11.00 h, soon after measurements began, reaching 4 and 3 times the levels of the previous day, respectively. Their ratio increased to 2.35 ± 0.18 suggesting that
levels peaked at 11.00 h, soon after measurements began, reaching 4 and 3 times the levels of the previous day, respectively. Their ratio increased to 2.35 ± 0.18 suggesting that  was being removed preferentially to
was being removed preferentially to  . The flow rate varied inversely to the concentrations of
. The flow rate varied inversely to the concentrations of  and
and  , showing the type of melt observed by Reference Johannessen and HenriksenJohannsen and Henriksen (1978). Cl− concentrations rose slowly to a maximum at mid-afternoon (35% above that of 12 July) when the net radiation was at its most intense and the meltwater flow rate was close to its maximum. H+ closely followed the
, showing the type of melt observed by Reference Johannessen and HenriksenJohannsen and Henriksen (1978). Cl− concentrations rose slowly to a maximum at mid-afternoon (35% above that of 12 July) when the net radiation was at its most intense and the meltwater flow rate was close to its maximum. H+ closely followed the  and
and  variation, peaking (at a pH of 4.3) at 11.00 h. Na tended to follow the same pattern but also reflected the Cl− levels.
variation, peaking (at a pH of 4.3) at 11.00 h. Na tended to follow the same pattern but also reflected the Cl− levels. -
14 July: the morning was dominated by low cloud and drizzle, the precipitation amount (<0.05 mm) being too small for analysis. Cl−, Na+ and H+ were much the same as the previous evening but
 and
and  had dropped back to their levels of 12 July. When the cloud cleared at 13.00 h meltwater concentrations immediately soared,
had dropped back to their levels of 12 July. When the cloud cleared at 13.00 h meltwater concentrations immediately soared,  and
and  values increasing five-fold while the pH dropped from 4.6 to 4.15 (a three-fold increase in H+) coinciding with a rapid increase in the flow rate (Fig.4). Na+ doubled while Cl− once again showed a more sluggish and variable response.
values increasing five-fold while the pH dropped from 4.6 to 4.15 (a three-fold increase in H+) coinciding with a rapid increase in the flow rate (Fig.4). Na+ doubled while Cl− once again showed a more sluggish and variable response. -
15 July: a similar pattern to 13 July, except for the decrease in Cl− from the high values established the previous day.
The overall pattern is one where ![]() and
and ![]() peak early in the daily melt, eluting quickly from the snowpack in the first meltwater fraction when flow rates were increasing. H+ follows a similar pattern. Over the four days,
peak early in the daily melt, eluting quickly from the snowpack in the first meltwater fraction when flow rates were increasing. H+ follows a similar pattern. Over the four days, ![]() and
and ![]() in the meltwater have a correlation of 0.98, while the correlation coefficient between
in the meltwater have a correlation of 0.98, while the correlation coefficient between ![]() and H+ is 0.94. The 99% confidence level for 30 degrees of freedom is 0.45. The slowly eluting ions, Na+ and Cl−, are well correlated with a coefficient of 0.74 (sea-salt is a significant constituent of maritime precipitation) but it is also clear from the correlation matrix (Table II) that a complex interrelationship exists between all five ions shown.
and H+ is 0.94. The 99% confidence level for 30 degrees of freedom is 0.45. The slowly eluting ions, Na+ and Cl−, are well correlated with a coefficient of 0.74 (sea-salt is a significant constituent of maritime precipitation) but it is also clear from the correlation matrix (Table II) that a complex interrelationship exists between all five ions shown.
Table II Correlation Matrix for Five Major Ions in Meltwater

An average charge balance for different periods of the melt sequence (Table III) reveals a large excess of positive charge throughout the melt. Even assuming the error of 0.2 pH units suggested by Reference McQuaker, Kluckner and SandbergMcQuaker and others (1983) as common in field measurements of weak unbuffered systems such as these, there are too few anions, by a factor of more than 2, to balance the hydrogen ions. Further laboratory analyses have failed to locate a further major anion missed by our initial analyses and we conclude that the absolute accuracy of our field pH measurement must be in error by more than 0.2 pH units (~0.5 pH). Errors of this magnitude in pH determinations are common as confirmed by the WMORPS (1978) study on the accuracy and reproducibility of rain-water pH and acidity. The relative pH values reported here were, however, repeatable and internally consistent, and show the general trends in H+.
Table III Average Ion Concentrations (μeq 1−1) in the Meltwater Over Different Periods

The ion concentrations of the meltwater suggest an elution order of ![]() ,
, ![]() , H+, Na+, Cl− (similar to that found by Reference Brimblecombe, Tranter, Abrahams, Blackwood, Davies and VincentBrimblecombe and others (1985) in their Scottish catchment) although it must be pointed out that some melting had occurred before this experiment and that this order may not be that of the first melt. Ca2+, Mg2+ and K+ concentrations were close to the limits of detection and so have not been included in the elution order. The relative depletion of ions between cores collected on 8 and 15 July may be a better indicator of the order of preferential elution. These cores suggest the order Ca2+,
, H+, Na+, Cl− (similar to that found by Reference Brimblecombe, Tranter, Abrahams, Blackwood, Davies and VincentBrimblecombe and others (1985) in their Scottish catchment) although it must be pointed out that some melting had occurred before this experiment and that this order may not be that of the first melt. Ca2+, Mg2+ and K+ concentrations were close to the limits of detection and so have not been included in the elution order. The relative depletion of ions between cores collected on 8 and 15 July may be a better indicator of the order of preferential elution. These cores suggest the order Ca2+, ![]() K+, Mg2+,
K+, Mg2+, ![]() , Na+, Cl− (see Table I).
, Na+, Cl− (see Table I).
The charge contributions of the ions in the meltwater and the correlation coefficients between ions suggest (a) that at times of low concentrations more H+ may be associated with the Cl− than with both ![]() and
and ![]() , while (b) at higher concentrations the additional H+ may be dominantly associated with
, while (b) at higher concentrations the additional H+ may be dominantly associated with ![]() and
and ![]() . However, because of the difficulties in the absolute pH determinations and the lack of
. However, because of the difficulties in the absolute pH determinations and the lack of ![]() measurements, these suggestions remain speculative.
measurements, these suggestions remain speculative.
3.3 Meltine experiment 1
Melting was completed in a single sequence, with the final snow melting after 14 h. Meltwater was collected in approximately 70 ml samples from each of the two melt exits. The central exit is referred to as exit C and the outer peripheral exit as exit P (Fig.2). Meltwater was collected from exit P after 4.5 h but collection from exit C began much later (after 6.8 h). The rate of meltwater collection was variable, due partly to the problem of keeping the lamp a constant distance above the ablating snow surface and partly because the percolation of the meltwater through the snowpack is a non-uniform process, the water moving along preferred channels or ducts rather than down the full cross-section of pack as a water front (Reference ColbeckColbeck 1972, Reference FosterFoster 1978, Reference HibberdHibberd 1984). The ionic concentrations for the anions and cations normalized to the bulk concentration at the beginning of the experiment are plotted in Figure 5 as a function of the total volume eluting through each exit. The time in hours is indicated on each graph so that the melt concentrations at any particular time can be compared. A total of 4.3 1 of meltwater were collected from exit P and 3.5 I from exit C.

Fig.5 Variation in the concentrations of selected ions for the first melt experiment from exit P (upper) and exit C (lower). The heating lamp was switched on at 07.00 h.
The meltwater concentrations from the two exits are Strikingly different in several respects and suggest that the processes occurring within a snowpack during melting may be quite complex. Meltwater from exit P, which collects water from the sides of the cylinder, shows a steady decrease in the total ionic strength with time, reaching the bulk concentration after 9 h (at 17.00 h) and dropping to less than 25% of bulk after 13 h. Meltwater from exit C, starting more than 2 h after exit P dropped to bulk at 7 h (at the third 70 ml sample from this exit) and to 25% of bulk only 30 min later.
The initial order of elution of the ions from the two exits is given in Table IV together with rank orders of the eight major ions. The sequences show some differences, particularly for the elution of ![]() , but both show rapid elution of K+, Mg2+ and
, but both show rapid elution of K+, Mg2+ and ![]() and slow elution of Na+ and Cl−, a pattern similar to that recorded in the Norwegian field experiment. The difficulties in the measurement of H+ have precluded its inclusion in these two graphs but in the first melt fraction from both exits H+ was less than in the bulk snow (by a factor of between 1 and 2).
and slow elution of Na+ and Cl−, a pattern similar to that recorded in the Norwegian field experiment. The difficulties in the measurement of H+ have precluded its inclusion in these two graphs but in the first melt fraction from both exits H+ was less than in the bulk snow (by a factor of between 1 and 2).
Table IV Ion Concentrations in the First Melt Fractions from the Two Laboratory Melt Experiments for Exits P and C (Concentrations are normalized to the bulk snow concentrations and rank orders of preferential elution shown.)

3.4 Melting experiment 2
In order to reproduce some of the diurnal effects noted in the Norwegian field experiment a second melting experiment was performed. The snow used was from the same drift as the first experiment but during the storage period of nearly a year the snow had taken on a much more crystalline structure, consisting of small crystals about 1 mm in diameter, and had the texture of the snow/firn often encountered deeper in a snowpack in spring. Melting was completed over two days with a night between. The meltwater concentrations from the exits P and C, normalized to that of the bulk snow, are shown in Figure 6. The first-melt concentrations are given in Table IV, along with their rank orders suggesting the sequence of preferential elution.

Fig.6 Variation in the concentrations of the same ions as shown in Figure 5 for the second melt experiment. Melting completed over 2 days, the lamp being switched on at 07.00 h each day. On the first day the lamp intensity was reduced to 35% at 15.00 h and switched off at 16.00 h.
Water was collected first from exit C (as opposed to exit P in experiment 1) within one hour of the heat lamp being switched on, but, after about 90 ml had been collected, there was a hiatus of 4 h until 13.00 h. From 13.30 to 15.00 h concentrations were very close to that of the bulk, representing a period of equilibrium melting, i.e. total melting of the near-surface snow (from which most ions had already been removed) and initial preferential melting of snow further down the column. The intensity of the heat lamp was reduced to 35% at 15.00 h and the ionic strength of the meltwater increased (as the meltwater flow rate dropped) and exhibited the same preferential elution as at the beginning of the melt. The lamp was switched off at 16.00 h and collection ceased at 16.30 h. During the next day melting continued, but after an initial sample with relatively high concentrations the meltwater collected at exit C had very low concentrations (with the exception of Na+ and Cl−, which stabilized at 30 and 40% of bulk respectively). In total, 3.2 1 of meltwater were collected from exit C.
Exit P showed quite a different pattern with concentrations increasing from 11.30 h, when the first melt was collected, to 15.00 h when collection stopped. The order of elution remained remarkably constant throughout the first day and was similar to that of the first-melt fraction. In contrast to exit C, there was very little melt collected from P when the intensity of the lamp was decreased. During the following day concentrations gradually decreased although there was an extended period from 09.00 to 11.00 h when they were close to those of the bulk.
4 Conclusions
The field and laboratory experiments show that fractionation (Reference Johannessen and HenriksenJohannssen and Henriksen 1978) and preferential elution (Reference Davies, Vincent and BrimblecombeDavies and others 1982) are important processes in determining the concentration of meltwater run-off from a melting snowpack and that the simple analysis of the chemical composition of bulk snow cannot be directly used as a surrogate for meltwater, either for absolute or for relative ion concentrations, However, the patterns of preferential elution and fractionation exhibited in these experiments may enable some conclusions to be drawn about the likely meltwater composition if the bulk snow composition is known (both chemically and physically) although more experiments are needed to verify the results shown here.
The order of preferential elution of the ions in a snowpack is very clear in some respects and much more confused in others. Cl− is consistently the most slowly eluted ion and Na+ is almost as slow. This is interpreted as being due to the importance of sea-salt as condensation nuclei in the atmosphere; much Cl− and Na+ will be trapped in the interior of ice crystals during freezing and only mobilized slowly with bulk melting. If the order of preferential elution is due predominantly to the position of the ions within, or attached to the outside of, the snow crystal (or, in the case of older snow or firn, to the ice crystal) then the order will vary depending on the atmospheric history of the snow. It will depend on the initial condensation and freezing of water vapour in the air-mass, and on the subsequent scavenging during transport and during the precipitation processes. Ion species associated with atmospheric pollution such as ![]() and
and ![]() could be expected to be attached to the surface of the snow crystals and thus be readily available for removal early in the melt process. In addition to this simple physical view of fractionation and of the phenomenon of preferential elution, the chemical properties of the individual ions will influence the ease with which they can be removed from the snowpack but it is not possible from the simple experiments described here to differentiate between the relative importance of the physical and chemical processes.
could be expected to be attached to the surface of the snow crystals and thus be readily available for removal early in the melt process. In addition to this simple physical view of fractionation and of the phenomenon of preferential elution, the chemical properties of the individual ions will influence the ease with which they can be removed from the snowpack but it is not possible from the simple experiments described here to differentiate between the relative importance of the physical and chemical processes.
The position of H+ in these experiments is uncertain. Although the relative H+ concentrations are well-defined, the absolute accuracy of the measurements is not. The field data suggest that the H+ ion is quite closely associated with the ![]() and the
and the ![]() ions and is eluted with these ions, although how much melting had occurred before these samples was unknown. The laboratory experiments show H+ as being a slowly eluted ion, in contrast to the field data and at variance with the results of other studies (Reference Davies, Vincent and BrimblecombeDavies and others 1982, Reference Brimblecombe, Tranter, Abrahams, Blackwood, Davies and VincentBrimblecombe and others 1985).
ions and is eluted with these ions, although how much melting had occurred before these samples was unknown. The laboratory experiments show H+ as being a slowly eluted ion, in contrast to the field data and at variance with the results of other studies (Reference Davies, Vincent and BrimblecombeDavies and others 1982, Reference Brimblecombe, Tranter, Abrahams, Blackwood, Davies and VincentBrimblecombe and others 1985).




 ,
, 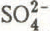 Cl
Cl









