11.1 Introduction
Medical products and devices make up one of the ‘building blocks’ of the World Health Organization (WHO) framework for analysing health systems. Safe and effective medicines, vaccines and medical devices are fundamental to a functioning health system. However, health systems deal with many challenges in trying to ensure that such products are available, affordable, effective and safe and used appropriately (World Health Organization, 2018). This chapter analyses the role of medical products in the development of the Malaysian healthcare system since independence 60 years ago. Medical products as discussed in this chapter include modern (allopathic) pharmaceuticals (both prescription and non-prescription), vaccines, health supplements and traditional medicines but do not cover medical devices. Medical products confer enormous benefits to health. However, they also have potential harmful effects, either through their inherent properties or through misuse and abuse. Therefore, this chapter addresses concerns about medical products’ access and affordability, alongside the aspects of safety and quality.
11.2 The Scene at Independence
Malaysia inherited a system in which traditional medicines were used side by side with modern (allopathic) medicines. Traditional medicines mostly originated from medicinal plants and had their roots in Malay, Chinese or Indian traditional medicine practices (Reference HarunHarun, 2006; Reference HeggenhougenHeggenhougen, 1980; Reference Tate, Khoo and SelvamanyTate et al., 2005). The largely rural population, who had very limited access to modern medical care, depended heavily on traditional medicines. Almost all allopathic medicines were imported from Britain through the British Crown Agents and delivered to the public either through the public sector health service or private sector doctors (general practitioners) or sold in private pharmacies (Reference HarunHarun, 2017). As described in earlier chapters, these health services were largely urban-based. Although there was legislation to regulate practitioners of allopathic medicine, there was no legislation or system to oversee traditional medical practitioners and traditional medical products. During the years immediately before independence, there was little awareness among the public regarding the safety and efficacy of pharmaceuticals.
Furthermore, opium addiction was a serious problem. Therefore, the government enacted several laws (Box 11.1) that sought to:
ensure that potentially toxic medicines and substances were adequately controlled and handled only by adequately trained healthcare professionals;
protect society from adulterated or unwholesome drugs, drugs deleterious to health and counterfeit drugs; and
prevent the public from being misled, resulting in the wrong use of medicines, ignorance of toxicity or ineffective treatment for life-threatening illnesses.
11.3 The First Phase of Evolution (1960–1970s): Focus on Increasing Access
11.3.1 The Driving Forces
Earlier chapters discussed how the first couple of decades following independence focused on increasing access to healthcare for the rural population through integrated development of the health sector within the broader socio-economic development of the country. A key component of the healthcare system was the supply of medicines free of charge to patients who attended public sector facilities. The budget of the Ministry of Health (MoH) bore the cost of these drugs. The number of health facilities, particularly in rural areas, increased rapidly (Chapters 3 and 4), and national programmes for controlling communicable disease spread throughout the country (see Chapter 6). All of these required a reliable supply of medicines and vaccines. Therefore, the initial focus was on the import, storage, distribution and dispensing of medicines (Pharmaceutical Services Programme, 2013).
11.3.2 Import, Production and Purchase of Medicines
For the first 15 years, most medicines were imported, and the Crown Agents had a near monopoly on the import of medicines and vaccines for public sector facilities (Federation of Malaya, 1962; British Resident Selangor, 1903). Subsequently, local agents took over this role. However, parallel import of a patented product from an alternative source was allowed, and this provided a less costly alternative source for a patented product. A few multinational pharmaceutical companies also imported and distributed medicines to the few private pharmacies and wholesalers, which in turn supplied them to private medical practitioners and public sector facilities.
The public pharmaceutical sector initially focused on developing logistics to ensure supply to the rapidly expanding network of healthcare facilities. The MoH constructed a large central Government Medical Store (GMS) that became responsible for acquiring supplies from agents and in turn supplying medicines to all MoH facilities through a transport network. Hospitals and state stores submitted invoices and obtained most of their medicines from the GMS (Box 11.2).
Box 11.2 Management of the flow of medicines in the MoH system to ensure uninterrupted supply at the front line
The GMS operated on a trust account of RM 40 million, with which it bought its initial stock.
State stores and hospitals received funds through their budget and used it to purchase supplies from the GMS.
If the GMS was unable to meet a request for supply, facilities could purchase a limited amount from the private sector.
Meanwhile, the GMS progressively developed the capacity for manufacturing medicines, and by 1970, it manufactured more than 150 types of pharmaceutical products, including tablets, large-volume intravenous fluids, injections in ampoules (including morphine and pethidine) and galenicals.1 A few large hospitals supplemented the GMS production capacity by manufacturing intravenous solutions and galenicals for their own use. There were only a few private pharmaceuticals manufacturers, such as Glaxo and Sterling Drugs, which were foreign companies with manufacturing plants in Malaysia (B. Yeap, recollections of early pharmacy services in Malaysia, personal communication to Thomas Paraidathathu, 2016). Moreover, the Institute of Medical Research (IMR) also produced a range of vaccines for a time (Reference Ramanathan, Cheah and DonderoRamanathan et al., 1976). The first Malaysian private pharmaceutical manufacturing company was the Malayan Pharmaceutical Factory (MPF) in Petaling Jaya. By the 1970s, more pharmaceutical manufacturers were set up in Melaka, Sungai Petani, Port Klang and Bangi (Malaysia Competition Commission, 2017). Thus, while hospitals and the GMS catered to the government hospitals, the private pharmaceutical manufacturers catered mainly to the private hospitals and clinics and sometimes, through tenders, also supplied the MoH.
11.3.3 Quality and Safety
During this phase of development, the system relied on the legislation that was already in place. However, from time to time, the MoH also issued circulars to ban the import and use of certain drugs or chemicals, such as phenformin, or re-classified them in the Poisons List to restrict their use. There was hardly any enforcement capacity; therefore, the MoH gradually built up the capacity for oversight. Box 11.3 shows the key landmarks in this process.
Prior to 1985, Malaysia did not require local registration of a medicine. Unless the MoH banned a product, importers or manufacturers could sell it in the country. Additionally, some manufacturers in the country used premises that were unsuitable for manufacturing and preparing medicines (e.g. garages and shophouses). They had a very poor understanding of the concept of good manufacturing practice (GMP). The quality of medicines and their compliance with pharmacopoeia standards were unknown (S. Selvaraja, experiences as a pharmacy regulator, personal communication to Thomas Paraidathathu, 2018). Furthermore, there were no inspections of manufacturing premises or licensing requirements from the MoH.
Also, as local registration of a product was not required, the Malaysian government suspected that some multinational pharmaceutical companies were ‘dumping’ their products in the country. For example, an analgesic product containing the drug dipyrone, which the US FDA2 had banned, was available in Malaysia.
Box 11.3 Landmarks in developing institutional capacity for oversight of pharmacy and pharmaceutical trade
1969: Government Medical Store (GMS).
1969: Pharmaceutical Chemistry Department in the MoH to cater for rising needs.
1970: National Pharmaceutical Control Laboratory (NPCL) for quality assurance of pharmaceuticals.
1976: Pharmacy Enforcement Unit in MoH to enforce legislation related to pharmacy and pharmaceutical trade.
1978: NPCL converted into National Pharmaceutical Control Bureau (NPC) to perform regulatory functions.
1984: Control of Drugs and Cosmetics Regulations in June 1984 ‘marked the dawn of the regulatory era’.
1985: Drug Control Authority under the chairmanship of the Director General of Health for ‘ensuring quality, safety and efficacy of pharmaceutical products prior to marketing’. The NPCB became its secretariat.
However, this period also witnessed the first baby steps for improving the quality of medicines dispensed at public sector facilities. Patients used to bring their own containers to public sector hospitals and clinics for oral solutions, lotions and eye drops.3 Such containers had inconsistent levels of cleanliness and sizes. Therefore, in 1979, the MoH decided to supply medicines in plastic containers, enabling standardised and hygienic packing. Moreover, pre-packing speeded up the dispensing process. In the private sector, quality and safety depended on the professional integrity of the medical profession and private pharmacies and their adherence to professional and legal requirements to prevent wrongful use of prescription medication. In 1983, inspired by the WHO initiative on essential drug lists, the MoH produced its own Blue Book, listing essential drugs for MoH facilities. This formed the basis for standardising the availability of medicines at each level of the facility in MoH services.
11.3.4 Prescription, Dispensing and Human Resources
During the first decade after independence, the very few Malaysian pharmacists trained mainly in Singapore, Australia or the United Kingdom. At the start of the second decade after independence, a local university established a training programme, but its output was limited. As population growth was relatively rapid, the ratio of pharmacists to population remained low. Dispensing medication was the responsibility of medical assistants4 in the public sector facilities and of clinic assistants under the supervision of doctors in private sector clinics. To address the need for personnel, particularly for hospital pharmacies, an allied health profession of dispensers was introduced, with one year’s training in a newly established Dispensers Training School followed by on-the-job training at hospitals. Gradually, the dispensers replaced medical assistants for dispensing in the public sector facilities (Pharmacy and Supplies Programme, 1996; G. Singh, experiences as a dispenser in the early years, personal communication to Thomas Paraidathathu, 2012).
11.3.5 Outcomes
Although there is no direct information on the availability of and access to medicines and vaccines, the success in reducing vaccine-preventable deaths (see Chapter 4) and malaria (see Chapter 6) and the rapid increase in the utilisation of hospitals and clinics (see Chapters 4 and 5) suggest adequate access to and use of basic medicines and vaccines. As discussed in Chapters 4–6, the availability and use of effective vaccines and medicines contributed to the changing profile of hospital bed utilisation.
11.4 The Second Phase (the 1980s–1990s): Strengthening Quality and Safety
11.4.1 The Driving Forces
During the next two decades after independence, Malaysia experienced stable politics, continued economic growth with a few downturns, rapid urbanisation due to rural-to-urban migration, and a demographic and epidemiologic transition (see Chapter 3). The incidence of communicable diseases declined while the prevalence of non-communicable diseases increased. (see Chapters 3–6). Meanwhile, the advent of newer pharmaceutical products and international research on effectiveness and risks changed medical care practices. One example is the management of eclampsia of pregnancy, where the use of magnesium sulfate enabled nurses in more remote settings to initiate effective management without awaiting the availability of a doctor.
11.4.1.1 International Influences
The generic drug industry flourished around the world, and in response, multinationals differentiated their products as better quality and supported by strong research. At the same time, the issue of the increasing presence of counterfeit medicines on the market, especially in some African countries, highlighted the need for stricter regulatory controls and enforcement. While countries like the US, the United Kingdom, Germany and Australia had well-established drug regulatory agencies such as the United States FDA and the Australian Therapeutic Goods Administration (TGA), many developing countries did not have good regulatory control of medicines. The WHO encouraged developing countries to establish regulatory systems for medicines and to participate in international collaboration for sharing of information of reports of adverse drug reactions (ADR) and adulterated and counterfeit medicines (Reference Raranawijitrasin and WondermagegnehuRaranawijitrasin & Wondermagegnehu, 2002).
11.4.2 Import, Production and Sale: Quality and Safety
11.4.2.1 Strengthened Governance Capacity: Legislation and Regulatory Requirements
Concerns about drug efficacy, quality and safety made it imperative that the government take steps to strengthen the governance process. This included the enactment of legislation (Box 11.1), building laboratory capacity and human resource competence for pharmaceutical analysis and for educating manufacturers, monitoring compliance with regulations, and enforcement of regulations. The Control of Drugs and Cosmetics Regulations (CDCR) 1984 under the Sale of Drugs Act established the Drug Control Authority (DCA) and gave it powers. The legislation included two key requirements for pharmaceutical products prior to sale in Malaysia: first, the registration of pharmaceutical products, and second, the licensing of manufacturers and wholesalers and of products for clinical trials. The CDCR established criteria for manufacturing facility location and required suitably qualified personnel, such as pharmacists, chemists and biochemists, and proper quality control procedures, both during manufacturing and for the finished product.
Empowered by the CDCR 1984, the DCA assumed responsibility for ensuring that medicines were safe, efficacious and of suitable quality prior to sale in Malaysia. The DCA implemented three initiatives: first, prior to registration and subsequent sale, the DCA instituted a process that included laboratory testing according to established pharmacopoeias or other standards and the evaluation of a dossier submitted by the manufacturer or applicant (on behalf of the manufacturer). Applications for the registration of imported medicines had to be accompanied by a Certificate of Free Sale (CFS), Certificate of Pharmaceutical Product (CPP) or an equivalent certificate from a competent authority in the country of manufacture, thus addressing the issue of the dumping of products not sold in the country of manufacture. All registered medicines were identified by a unique registration number intended to increase consumer confidence in the product’s safety and efficacy.
11.4.2.2 Stronger Monitoring Complemented by Education and Information
Second, the DCA inspected manufacturers to ensure compliance with GMP guidelines published by the WHO (World Health Organization, 2011). In conjunction, the MoH provided education regarding GMP and established a system to provide reliable information to the public and healthcare providers regarding medical products.
Third, the DCA initiated a pharmacovigilance system through a voluntary reporting system, whereby healthcare professionals and members of the public were encouraged to report suspected ADR. Initially, the number of reports to the Malaysian Adverse Drug Reactions Advisory Committee (MADRAC) was not encouraging. Subsequently, the National Pharmaceutical Control Bureau introduced a number of initiatives such as letters of appreciation to encourage people, especially healthcare personnel, to submit suspected reports. Malaysia has been a participant of the WHO Adverse Drug Reactions Monitoring Programme since 1987 (Reference Raranawijitrasin and WondermagegnehuRaranawijitrasin & Wondermagegnehu, 2002), and reports of ADR are sent to the WHO Collaborating Centre for International Drug Monitoring in Uppsala, Sweden.
The introduction and enforcement of the CDCR is believed to have increased consumer confidence regarding the quality, safety and efficacy of medicines in Malaysia. A number of popular combination products that were available prior to 1985 are no longer available in Malaysia. They included products for which there was no good scientific rationale, such as Franol, which was a combination product for asthma that contained theophylline, ephedrine and phenobarbitone.5 Products without proven efficacy or with unacceptable risk-to-benefit considerations and low-quality, cheap generics became almost non-existent in the Malaysian market.
11.4.2.3 Production Evolved
As a result of the regulatory changes, in the 1990s, government hospitals ceased the bulk manufacturing of pharmaceutical products such as intravenous fluids, eye drops, mixtures and creams because of difficulty complying with GMP requirements.
Simultaneously, the national policy to privatise selected public sector activities reached the pharmaceutical sector. During the 1980s, the GMS had continued to manufacture and supply medicines to government institutions, and the annual turnover increased from RM 100 million in 1984 to RM 150 million by 1994 (R. Kumarasingham, recollections of early pharmacy services in Malaysia, personal communication to Thomas Paraidathathu, 2016). In 1994, the GMS was privatised, and soon, under the name of Pharmaniaga,6 it acquired other manufacturing plants, became a large, fully integrated healthcare company and took on manufacturing, logistics, distribution, sales, marketing and supply of equipment. Pharmaniaga received an initial 15-year concession (up to 2009) for supplying to MoH institutions 700 items on the Approved Products Purchase List (APPL) and set up an integrated information system for the MoH institutions to order and monitor their supply of medicines. Initially, there were complaints of steep increases in prices, but these issues appear to have diminished over time. Stock-outs or low stocks of medicines at public healthcare facilities occurred occasionally, and patients received smaller amounts of medicines and had to refill more frequently.
Meanwhile, the local pharmaceutical industry grew. By the 1990s, the number of pharmaceutical manufacturers increased to more than 50, and all complied with international GMP standards. They also began exporting to other countries. In 1990, Malaysia exported a total of about RM 70 million worth of medicinal and pharmaceutical products (SITC7 541) and medicaments (including veterinary medicaments) (SITC 542), which was equivalent to about 5% of the total chemicals export of the year (Pharmaceutical Society, 2002).
11.4.3 Purchase: Effectiveness and Cost of Medicines
In the face of the growing choice of available medicines, the need for rational choice became a priority, particularly considering the national policy of providing highly subsidised medicines almost free to clients in public sector healthcare facilities. In 1983, the MoH established a system for selecting medicine eligible for purchase by the MoH through the Ministry of Health Medicines Formulary (MOHMF). An MoH review panel comprising representatives from different clinical disciplines uses criteria such as efficacy, safety, budget impact and ethical considerations for selecting items from the MOHMF. Other methods of selection include multi-criteria decision analysis (MCDA). The review panel aims to ensure that selection is transparent, predictable and considers the value to the community of patients and the cost of a drug. For example, if the cost of a drug were so prohibitive that only a fraction of the patients who need it would receive it, it would not qualify for inclusion in the MOHMF. The MOHMF is updated regularly, and in 2017, there were 1,676 drugs in the list. Other healthcare providers in the public sector, such as teaching hospitals and the Ministry of Defence, have their own formularies.
11.4.4 Prescription, Dispensing and Human Resources
For the first 25 years of independence, there was only one pharmacy school in Malaysia, with a small output that was augmented by a trickle of pharmacists trained abroad. In 1990, there were only 1,239 registered pharmacists (less than 1 per 10,000 population) (Ministry of Health Malaysia, 2000). The rapid expansion of the pharmaceutical sector was accompanied by the concentration of the available pharmacists in the private sector. In 1996, 77% of pharmacists were in the private sector (Ministry of Health Malaysia, 2000).
Due to the limited availability of pharmacists, their traditional role in the MoH during the 1950s to 1980s was in functions related to improving medicine quality and safety, enforcing standards for medicines and in the procurement and supply of medicines for patients. In the public sector, dispensing remained in the hands of assistant pharmacists, and in the private sector, largely in the hands of clinic assistants and their supervisory doctors. With the gradually increasing availability of human resources, pharmacists in MoH hospitals began to advise on individualised drug therapy, re-constituting cytotoxic drugs, providing medication counselling and developed drug information systems in hospitals (Ministry of Health Malaysia, 2002). By 1995, three other pharmacy programmes had begun in Malaysia, thereby boosting production output. The first graduates from these programmes began coming into the market by the end of the 1990s.
11.5 The Third Phase (2000s–2010s): Growing Concerns about Affordability
11.5.1 Import, Production and Sale: A Stable Regimen for Quality and Safety
The National Pharmaceutical Regulatory Agency (NPRA)8 succeeded in constructing a good regulatory framework for pharmaceuticals, and it gained international recognition. Co-ordination with customs authorities was strengthened to monitor consignments of products such as saccharin, beta-agonists, precursor and essential chemicals liable to be misused, for example in illegal traffic in narcotic drugs (Ministry of Health Malaysia, 2002). Additionally, the scope of monitoring extended to traditional products adulterated with poisons such as fenfluramine. In 2002, the NPRA became a member of the Pharmaceutical Inspection Co-operation Scheme (PIC/S), an international non-binding, informal co-operative arrangement between regulatory authorities in the field of GMP of medicinal products. This indicated that the NPRA adhered to internationally acceptable GMP inspection standards. Indirectly, it also validated the standard of the local pharmaceutical industry. The NPRA is also an active member of the ASEAN (Association of Southeast Asian Nations) harmonisation initiatives for the regulatory control of pharmaceuticals. In addition, Malaysia was accepted in 2013 into the Organisation of Economic Co-operation and Development (OECD) system for Mutual Acceptance of Data (MAD) in the assessment of pharmaceuticals. New medicines that are needed in Malaysia but that require more evidence of safety and efficacy are sometimes given conditional registration. Recently, a vaccine for dengue was given a two-year conditional registration. Phase IV clinical trials are still to be conducted, and therefore it has not been given full marketing authorisation.
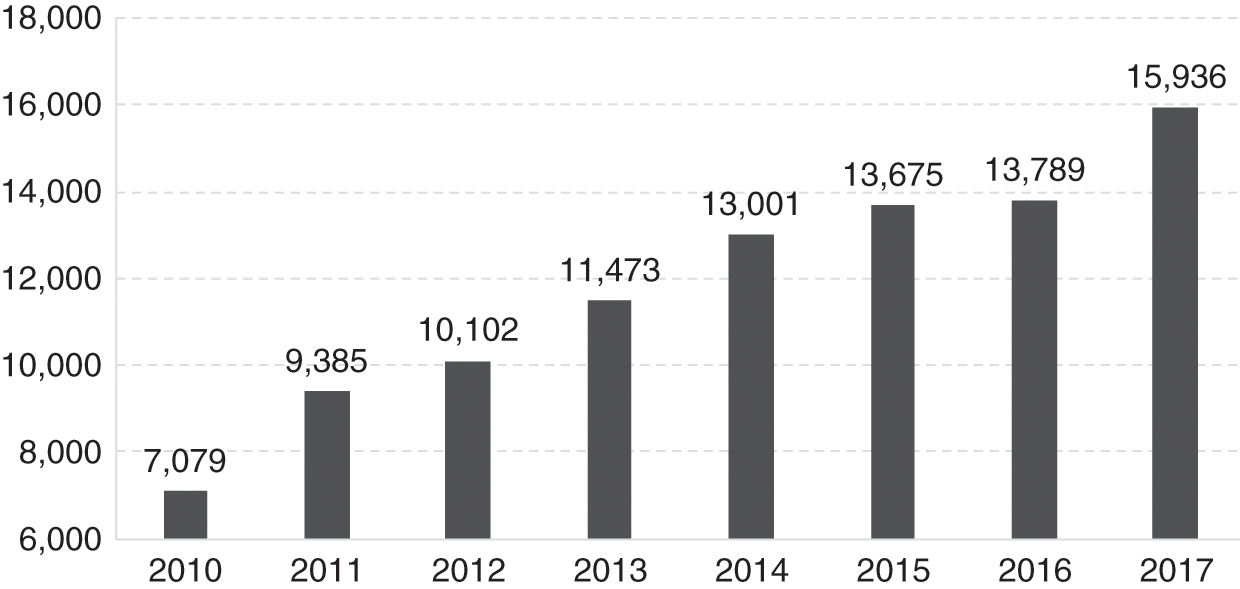
After an initial slow start during the 1990s, the reporting of ADR to MADRAC improved, accompanied by appropriate regulatory action by the DCA. Figure 11.1 shows the rise in the number of received reports from 2010 (7,079) to 2017 (15,936). The MoH conducted post-marketing surveillance of pharmaceutical products to ensure that registered products continued to adhere to stipulated standards of quality and conditions of use.
Counterfeit medicines amounted to less than 5% of all marketed pharmaceutical products. A significant contributor to this healthy scenario is the access to good-quality medicines at low or no cost at government healthcare facilities. The instances where counterfeit medicines were discovered involved medicines with high consumer demand such as Panadol and Viagra. Since 2005, a product-specific hologram was introduced as an additional safety feature for all registered drugs.
Advertisements.
In the 2000s, Malaysia adopted a national policy of promoting medical tourism. In response, the Medicine Advertisements Board (MAB) relaxed the conditions related to advertisements for medicines, provided improved guidelines and simplified the approval process for over-the-counter (OTC) drugs, functional medicines and health supplements. However, the industry is pushing for similar relaxations to the direct-to-consumer advertisement of controlled medicines, but this is not allowed. Simultaneously, the MoH strengthened efforts to provide reliable information to the public and healthcare providers through its centralised information centre and programmes such as Know your Medicines and Ask your Pharmacist. Meanwhile, the private sector took the initiative to produce the MIMS (Monthly Index of Medical Specialities), a reference publication for Malaysian medical professionals providing updated prescribing information and drug availability.
11.5.2 Supply Logistics, Prescription, Dispensing: Availability of Medicines
In the MoH system, logistics improved with the increased use of information technology that facilitated modernised inventory management and enhanced storage facilities. The volume of prescriptions in MoH facilities increased rapidly. For example, there was a 12% increase in one year during 2016–2017 (Ministry of Health Malaysia, 2017). Approximately 30–40% of these are repeat prescriptions for patients with chronic illnesses such as diabetes and hypertension who had prescriptions for 3–6 months’ duration. However, dispensing was monthly to monitor for any side/adverse effects, efficiently manage resources, avoid extensive stock holding and reduce medicine wastage resulting from improper storage or expiration. This dispensing practice caused inconvenience and travel costs to patients, congestion, and longer waiting times at pharmacy counters, particularly in the larger healthcare facilities. Several initiatives addressed this challenge. A digitalised Integrated Drug Dispensing System (Sistem Pendispensan Ubat Bersepadu, or SPUB) has enabled patients to obtain their prescriptions at any MoH facility and to do so through an appointment system that enables prior preparation of the prescription, thus drastically reducing waiting times. Other innovations have increased access and convenience. The drive-through pharmacy is a concept borrowed from the fast food and banking industries. The Medicines by Post scheme sends medicines to the patient’s home by a courier company for a fee of RM 6. The Locker4u scheme provides designated lockers, secured by a key/personal identification number (PIN), where patients can collect their medicines from hospitals at their own convenience. Patients who use these initiatives are very satisfied (98% satisfaction) with these services (Pharmaceutical Services Programme, MoH Malaysia, 2016).
As the availability of pharmacists increased, the scope of their service expanded, with more MoH hospitals providing pharmaceutical services such as parenteral nutrition, therapeutic drug monitoring, cytotoxic drug reconstitution, clinical and ward pharmacy services, and medication therapy adherence clinics and promoting quality use of medicine.
11.5.2.1 Human Resources
The production of pharmacists increased rapidly. The number of local pharmacy degree programmes increased rapidly to 22. The expansion was largely due to 17 institutions in the private sector; Chapter 8 discusses this phenomenon. The number of pharmacists per 10,000 population increased 4-fold within 14 years, namely from 1.02 in 2000 to 4.08 in 2014. The profile of the pharmacy workforce evolved rapidly (Figure 11.2). Two factors contributed to a rapid increase in the public sector workforce of pharmacists. First, in 2004, the government introduced a two-year period of compulsory service in the public sector for new graduates. Initially, there were concerns that this move would result in a shortage in the private sector, but it was expected that such a shortage would be short-lived, as market forces would continue to entice them to the private sector.
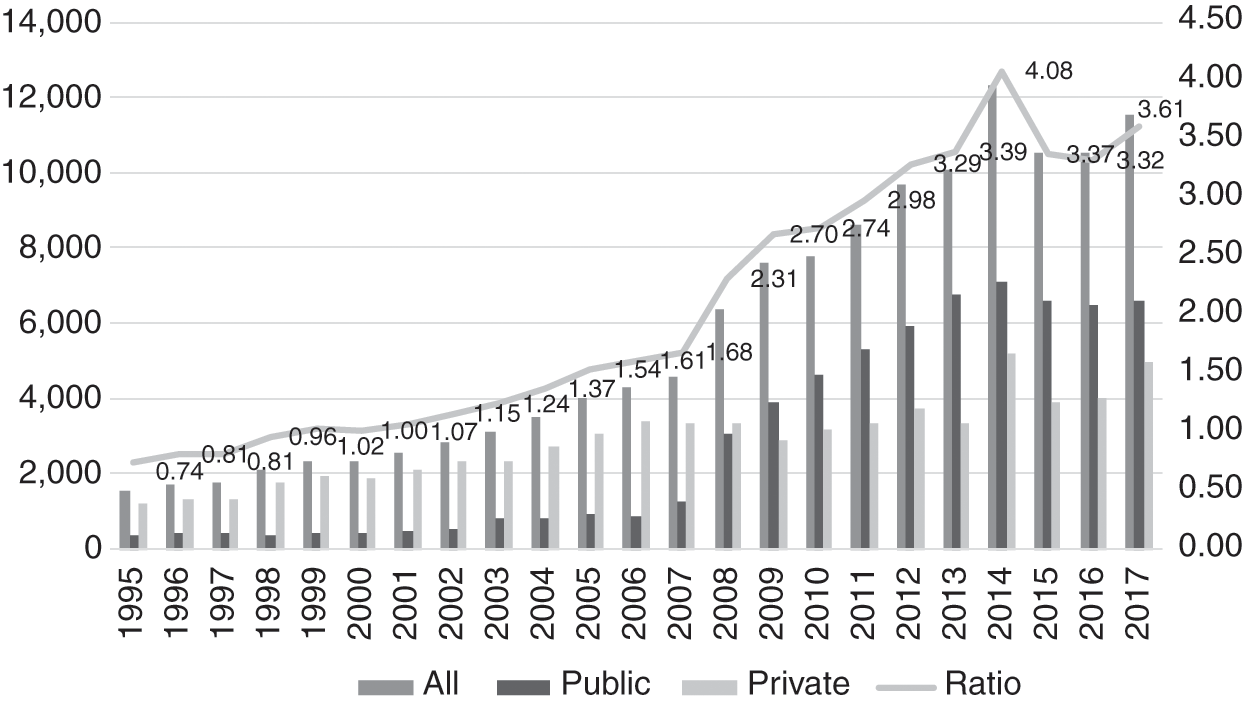
However, a second factor intervened. The government amended its civil service conditions, resulting in pharmacists receiving higher starting salaries and better opportunities for career advancement. As a result, more pharmacists chose to remain in the public sector, and by 2009, the numbers in the public sector overtook those in the private sector. The sudden influx of new graduates was similar to that experienced in the medical workforce, discussed in Chapter 8. Its consequences for the public sector were similar – several administrative adjustments were required to accommodate the new graduates, who required supervision and monitoring during the first years of their service prior to full registration.
Meanwhile, the number of pharmacy assistants continued to increase, rising from 1.01 per 10,000 population in 2002 to 1.87 in 2017 (Figure 11.3). Until 2012, they were almost exclusively in the public sector, when the numbers in the private sector began to increase gradually. By 2005, the number of pharmacists had overtaken that of assistant pharmacists.

The increased availability of pharmacists produced other changes. First, their role in the public sector started to expand gradually to cover direct services to clients. Second, the simultaneous influx of large numbers of new graduates, both medical and pharmacy, stretched the capacity of the public sector to absorb them to the limit. This resulted in a push from the public sector into the private sector, in contrast to earlier years, when the attraction of the private sector had caused movement of both professions out of the public sector. Some indication of the stress to the private sector is the ongoing controversy between the pharmacy and medical professions regarding the separation of prescription and dispensing functions in the private sector. Pharmacists argue that this would be in the best interests of patients in order for them to obtain cost-effective medicines and avoid unnecessary use of medications and their side effects. The counter-arguments from the medical profession include: (a) that the relative scarcity of private pharmacies in rural areas would limit access to medicines, and (b) that ‘patients go to pharmacies to buy medicines after consulting the doctor once, and then continue buying prescription-only medicines without a prescription’. General practitioners (GPs) further acknowledge that in the face of multiple challenges, including a fee schedule that has been unrevised for 27 years, ‘most GPs survive because the sale of medicines provides a small margin of profit’ (Reference PhilipPhilip, 2019). The economic factors underlying the controversy are evident.
The parallel development of dispensing practices in public healthcare facilities and private clinics provides a good illustration of path dependency in health systems. The lack of pharmacists in the early years of the health system (Section 11.3.4) resulted in other health personnel taking on the dispensing role. In public healthcare facilities, economies of scale kept the dispensing role specialised such that assistant pharmacists (Section 11.4.4) and then pharmacists were later able to take on this role. In private clinics, however, prescription and dispensing remained closely linked in the absence of policy or systemic incentives to separate these functions.
11.5.3 Affordability and Pharmaceutical Pricing
In October 2006, in line with WHO recommendations, the Malaysian National Medicine Policy (NMP) (Dasar Ubat Nasional, or DUNas) came into force. It covers all aspects of medicines. The objectives of DUNas are to improve the health outcomes of Malaysians through:
1. Promoting equitable access to essential medicines.
2. Ensuring the availability of safe, effective and affordable medicines of good quality.
3. Promoting quality use of medicines by healthcare providers and consumers.
Affordability is an important component of DUNas to ensure that cost does not become a barrier to equitable healthcare. To this end, DUNas encourages ‘Efforts … to promote healthy competition towards fair, transparent and sustainable cost-effective treatments’ (Ministry of Health Malaysia, 2012b).
Rising healthcare cost is a global phenomenon, albeit the rates grow differently by country. Malaysia’s medical inflation rates for 2019 were forecast to be about 5.7 times higher than that of general inflation (Aon, 2019). While there are many contributing factors to rising healthcare costs, higher drug costs is a significant factor. Drug affordability is a legitimate concern for patients with critical illnesses and those who require chronic therapy. Estimated conservatively, the health expenditure on medical goods in 2017 was 8%, or RM 4.55 billion of the national total health expenditure (Ministry of Health Malaysia, 2019b).
11.5.3.1 Public Sector
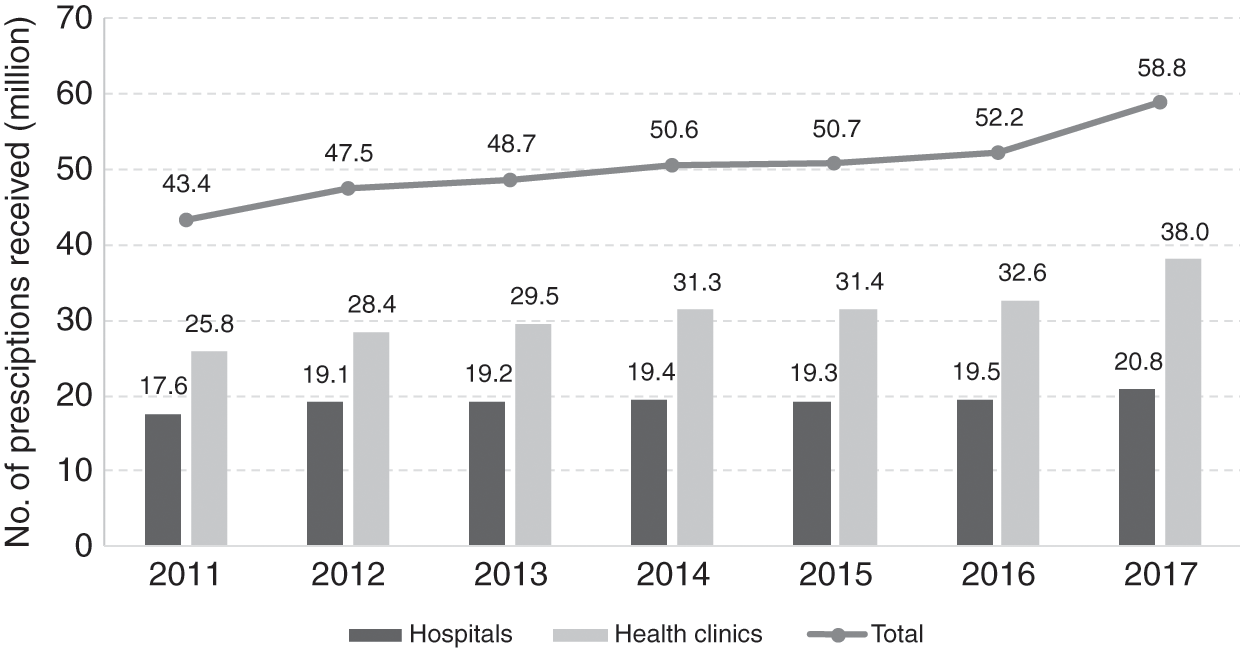
The public sector provides care for the majority of patients in Malaysia. For example, 70% of hospital admissions in 2017 were in public healthcare facilities. Hence the government budget allocation for medicine procurement is a concern. The total amount allocated for medicine procurement has remained about the same since 2014 (Figure 11.4) despite a yearly increase in public demand for drug treatment (Figure 11.5). In 2017, the allocation for medicine procurement for the MoH was about 10% (RM 2.38 billion) of the MoH operating expenditure.

Figure 11.4 MoH medicine expenditure, 2008–2017.
The government has to balance budget limitations against factors such as drug accessibility, value and (opportunity) cost and the rising demand for effective, quality and safe drug treatment. It does this through careful planning and procurement strategies.
The MoH practices an open tender policy for pharmaceutical products listed in the APPL (about 700 items determined by the MoH, which includes many items from the National Essential Medicines List) and the MoH National Tender (about 300 items). The MoH uses international best practices, for example, the WHO procurement process and workflows, for medicine procurement. Thus the process uses the indicative drug price based on market survey when calling for the open tender. This ensures that government central purchase obtains products that offer the best value. The MoH Procurement Board selects successful bidders after bids pass through the Technical and Financial Evaluation Committees. Only local purchase orders with a total value of less than RM 500,000 are exempted from open tender. Generally, generic medicines provide value while meeting budget constraints and public demand. Thus, in 2017, about 60% of drugs procured and dispensed were generics (30% local manufacture, 70% imported) (Pharmaceutical Services Programme, Ministry of Health Malaysia, 2018).
In its attempts to meet the increased demand for drug treatment, the government seems to have tried to keep the price it pays for drugs low to curb the escalation of its expenditure on drugs. However, the price the government pays for generic and originator drugs, although substantially lower than that charged in the private sector, has remained above the international reference median price (Table 11.1). Figure 11.5 shows that, from 2014 to 2017, despite no significant increase in its budget for medicine procurement, the MoH managed to provide an additional 8.2 million outpatient prescriptions. MoH management of budget disbursement in three-month staggered periods instead of annual disbursement to individual hospitals and clinics promotes prudent planning and more accurate forecast of local demand, thereby reducing the risk of drug shortage or wastage.
Table 11.1 Price comparisons in private sector outlets
| Generics | Originators | |||
|---|---|---|---|---|
| Public sector | Private sector | Public sector | Private sector | |
| Median price compared to international reference price (MPR) | 1.2 | 2.5 | 1.6 | 8.6 |
| Generics | Originators | |||
| Hospitals | Pharmacies | Hospitals | Pharmacies | |
| Mark-up in the private sector | 51% | 22.4% | 167% | 94.7% |
As described, in the development since the 1990s, Pharmaniaga, although a private entity and concessionaire, continues to play an important role in logistics and storage service provision to MoH health facilities nationwide, even if the request is made by a health facility in a remote area. In addition, it handles the open tender processes on behalf of the MoH and the Ministry of Finance (MoF). As a local generic drugs manufacturer, Pharmaniaga may also bid for government procurement tenders. As Pharmaniaga is responsible for handling the bids, the issue of potential disadvantage to other competitors has been considered. The response to this was that bids by Pharmaniaga must be submitted at least two weeks before the close of tenders.
11.5.3.2 Private Sector
Much expenditure on medical products is in the private sector. For example, the private sector spent RM 3.55 billion or 77% of the total medical goods expenditure9 in 2016, and there is a wide range of prices for medical products. Higher procurement prices due to the lack of economies of scale and high mark-ups by service providers or retailers10 are the principal contributory factors (Table 11.1).
As about 30% of inpatients use private hospitals, they pay a substantial price for medicines. The mark-up in private hospital prices is not transparent for patients or their insurance companies, and this asymmetry of information is a contributor to market failure. For certain life-saving and critical-care prescription drugs that are under patent protection, the implications are significant. For example, gefitinib, a cancer drug, cost 190.8 days of minimum wage per treatment in 2017, while trastuzumab, a breast cancer drug, cost 551.7 days of minimum wage, that is, more than 1 year of minimum wage (Pharmaceutical Services Programme, Ministry of Health Malaysia, 2018).
The cost of patented drugs is a barrier to access, and their generic competitors allow more patients to afford the drugs, hence resulting in increased sales. The government policy of favouring the lowest-priced generic drug procurement and the timely entry of generic drugs into the market has had strong effects in terms of drug affordability for patients. An illustrative example in Malaysia is that the originator pharmaceutical company was willing to reduce the price of trastuzumab11 by about 52% to win an MoH tender to compete with a local drug company that had also bid to supply a biosimilar drug.
In view of the potentially significant positive impact of generics on public expenditure on medicine, the Malaysian intellectual property patent law (Patents Act 1983) could be reviewed to expedite generic entry, for example, by reviewing patent criteria and the mechanism for allowing pre-grant and post-grant opposition. Furthermore, the introduction of Bolar-type12 provisions in regulatory approval would protect local manufacturers from harassment through threats of legal action. Under the government drug procurement public tender process, local generic drug manufacturers have a slight preference of a 10–15% higher price margin advantage, given the experience that local producers can provide a more consistent and reliable drug supply.
Although Malaysia has price controls on a number of food items and petrol, it has thus far not imposed price controls on medicines. By and large, medicines are affordable, especially since the government provides high-quality, safe and effective medicines primarily through the MoH at almost no cost to the patient. The presence of a competitive local generic manufacturing industry certainly helps in ensuring that generic products remain affordable. However, the challenge is in ensuring that imported patented innovator products are also affordable. If costs become prohibitive for the government to purchase patented drugs for a large number of patients, it can use provisions under the agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) to make such medicines available at a cheaper price for public non-commercial use.
In addition, Malaysia is one of the initial six co-sponsoring nations that proposed a resolution at the 72nd World Health Assembly that makes the pricing of medical products more transparent, and the resolution was adopted on 28 May 2019. The price information inputs are the cornerstone for the effectiveness of any future policies on drug price monitoring and control.
11.5.4 The Case of Right to Government Use
In early 2000, the costs of certain antiretroviral (ARV) drugs that were imported and under patent were so high that it limited the country’s ability to provide treatment to HIV-infected patients requiring it. In spite of negotiations, a suitable price could not be agreed upon. In 2003, the Malaysian government exerted rarely used powers to acquire the drugs through a tender to a local pharmaceutical company to import and supply selected generic versions of patented ARV drugs manufactured by the Indian company Cipla. This was possible by using a provision provided under the TRIPS agreement in line with the Doha Declaration 2001 by members of the World Trade Organization (WTO) and the provision of the Rights of Government under the Patents Act 1983 (World Trade Organization, 2001). The medication would be solely for the use of patients managed by MoH facilities. On completion of the ‘government use’ licensing period, the MoH was able to negotiate with the multinational companies for better prices and therefore no longer required the use of special provisions. A similar situation arose in 2017 for medication for hepatitis C; it is the subject of Case Study 12.1 in Chapter 12.
The government has indicated that it will not hesitate to use this mechanism in public health emergency situations where a large number of patients do not have access to affordable treatment. Malaysia is the first and one of only a few countries in the world to exercise this right to provide access to a patented product as part of its public health policy.
Box 11.5 System observations: the need for an equitable international system for drug development and public good
The cost of medication and other medical technologies is a limiting factor in the advancement of universal healthcare (UHC). Managing the tension between public goods and private incentives to develop and produce medical technologies is an ongoing challenge that transcends national boundaries, as seen here and in Case Study 12.1. There are significant provisions for low-income countries under international law, though obstacles to exercising these provisions exist and need to be addressed. An equitable framework for cost sharing between middle-income and high-income countries remains absent. The current approach of case-by-case resolution is opaque, drains resources and favours countries with political or economic leverage, thus creating inequitable outcomes.
11.5.5 Pharmaceutical Trade Sector in Malaysia
Pharmaceutical products are important trade items for Malaysia. In 2017, their trade amounted to RM 6.45 billion or 0.36% of total trade. However, the import of pharmaceutical products was about six times larger than the total export value (Figure 11.6). This created a trade deficit of RM 4.6 billion in 2017. In accordance with the government’s Economic Transformation Programme, a project was implemented in 2010 to provide incentives for the local production of generics for export. Subsequently, the sales of generic and patented medicines as a percentage of prescription drugs have seen a reversal, with generics overtaking patented drugs in 2013 (Malaysia Competition Commission, 2017). The local pharmaceutical manufacturing sector started to pick up and saw a 63.7% increase in the export value from 2013 to 2017 compared to a 31.3% increase in the value of imported medicines (Figure 11.6). The increased export value for formulated products (medical products) for human use (Table 11.2) contributed 81.0% of all pharmaceutical products export in 2017.
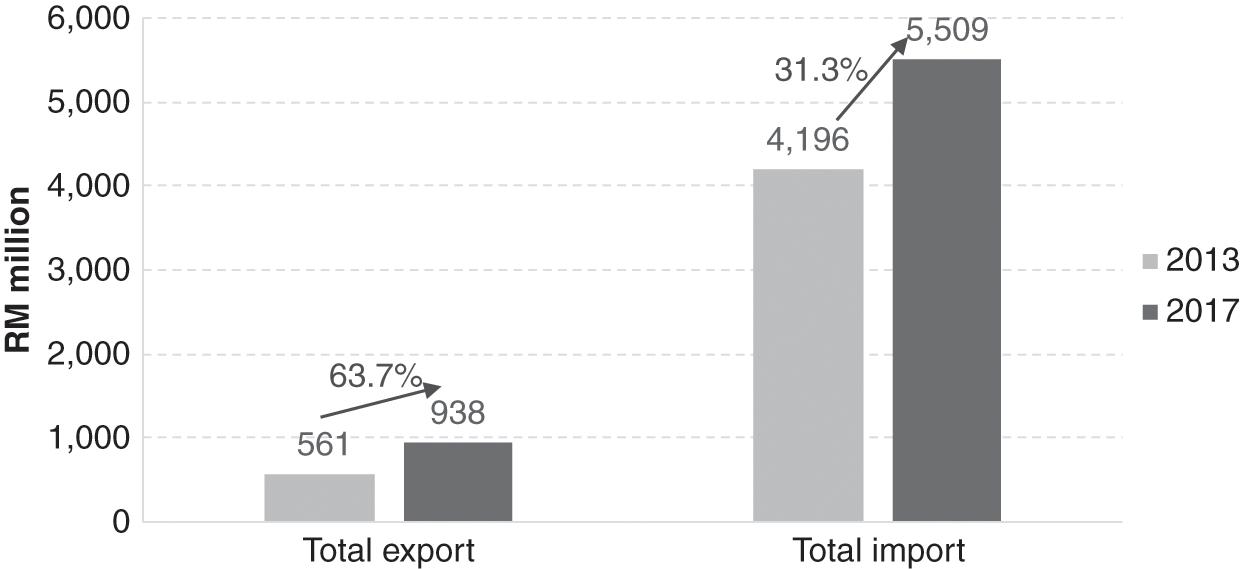
Table 11.2 Export and import value of pharmaceutical products to Malaysia by product category, 2013–2017
| No. | Product category | 2013 | 2017 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Export | Import | Export | Import | ||||||
| Value (RM million) | % | Value (RM million) | % | Value (RM million) | % | Value (RM million) | % | ||
| 1 | A1: Formulated products for human use | 453.1 | 80.8 | 3,548.9 | 84.6 | 760.5 | 81.0 | 4,551.1 | 82.6 |
| 2 | A2: Formulated products for veterinary use | 6.1 | 1.1 | 80.0 | 1.9 | 25.2 | 2.7 | 66.1 | 1.2 |
| 3 | A3: Vaccine products for human and veterinary use | 6.1 | 1.1 | 231.8 | 5.5 | 11.6 | 1.2 | 415.3 | 7.5 |
| 4 | B1: Vitamins | 34.0 | 6.1 | 162.7 | 3.9 | 86.3 | 9.2 | 262.7 | 4.8 |
| 5 | B2: Antibiotics | 61.8 | 11.0 | 173.0 | 4.1 | 54.9 | 5.8 | 214.1 | 3.9 |
| Total | 561.0 | 100.0 | 4,196.4 | 100.0 | 938.5 | 100.0 | 5,509.2 | 100.0 | |
11.6 Key Messages from Malaysia’s Experience
11.6.1 What Went Well?
The medical products subsystem at various times in development:
◦ Responded to demands from rapidly expanding health services and more variety and higher costs of internationally available medical products.
◦ Dealt with hazards and stresses such as trade in illicit and dangerous drugs, dumping of poor-quality and unsafe medicines, misuse and abuse of allopathic and traditional medical products, internationally rising costs of pharmaceuticals, and patent protection.
◦ Served its purpose despite facing constraints, including human resources and budgetary limitations.
The health system successfully improved access, safety and quality of imported and local products, quality of local production, and cost control through incremental steps in strengthening:
◦ logistics and production capacity,
◦ legislation, monitoring and enforcement,
◦ education and negotiation.
11.6.2 What Did Not Go So Well?
The inability to moderate the high and increasing cost of medical products in the private sector.
11.6.3 Trends and Challenges
Challenges in traditional medicines and health supplements:
◦ Adulteration
◦ Inappropriate marketing and use
◦ Sale as health foods to bypass regulation of medical products
Pressure from the pharmaceutical industry for patent protection.
Implementation of price control tools.
National formulary for all public sector health facilities to enable them to have economies of scale in purchasing and to have uniform policies such as antibiotic policies.