1.1 Introduction
Although the assessment and management of ovarian cysts in non-pregnant women has been studied extensively, unfortunately the same does not apply to the pregnant population. It is difficult to be certain about the exact incidence of ovarian cysts complicating pregnancy, and rates reportedly vary between 0.05–6% [Reference Schwartz, Timor-Tritsch and Wang1]. Their prevalence decreases with advanced gestational age, likely due to spontaneous resolution of physiological ovarian cysts.
Historically, ovarian cysts were diagnosed prenatally if they were large enough to be palpated, or they might be an incidental finding during a Caesarean section. The widespread use of ultrasound in early pregnancy and antenatal surveillance has led to an increase in the diagnosis of ovarian cysts during pregnancy. Fortunately, most of them are benign and will resolve spontaneously during the antenatal period. Nevertheless, even benign cysts can generate symptoms and become a source of anxiety, both for patients and for their physicians. In addition, a small proportion of ovarian cysts in pregnancy (about 1–5%) will carry some malignant potential [Reference Webb, Sakhel, Chauhan and Abuhamad2, Reference Schmeler, Mayo-Smith and Peipert3]. Hence, early identification, appropriate diagnostic work-up and optimal management are necessary, of course taking into consideration the complexities arising from the pregnant status of the patients.
This chapter serves to summarise current evidence for the assessment and management of ovarian cysts in pregnancy. Key concepts such as counselling, optimal time to operate and post-natal follow-up are also discussed.
1.2 Types of Cysts
Similarly to non-pregnant women, ovarian cysts identified in pregnancy can be broadly divided into three categories: benign, malignant and borderline cysts. About 95–99% of them are benign, while malignant (most commonly germ cell, sex cord stromal and epithelial tumours) and borderline cysts (predominantly serous and mucinous borderline tumours) account for approximately 1–5% and 1–2% of the ovarian cysts diagnosed in pregnancy, respectively [Reference Webb, Sakhel, Chauhan and Abuhamad2, Reference Schmeler, Mayo-Smith and Peipert3].
Most ovarian cysts are functional in nature with the corpus luteum being the most commonly encountered cyst in pregnancy [Reference Condous, Khalid, Okaro and Bourne4]; functional ovarian cysts may also represent simple follicular or haemorrhagic cysts. The most usual benign, non-functional cysts are endometriomas, mature teratomas (dermoid cysts) and serous or mucinous cystadenomas or cystadenofibromas. The above cysts demonstrate distinct ultrasound features and are usually readily identifiable by an experienced sonologist.
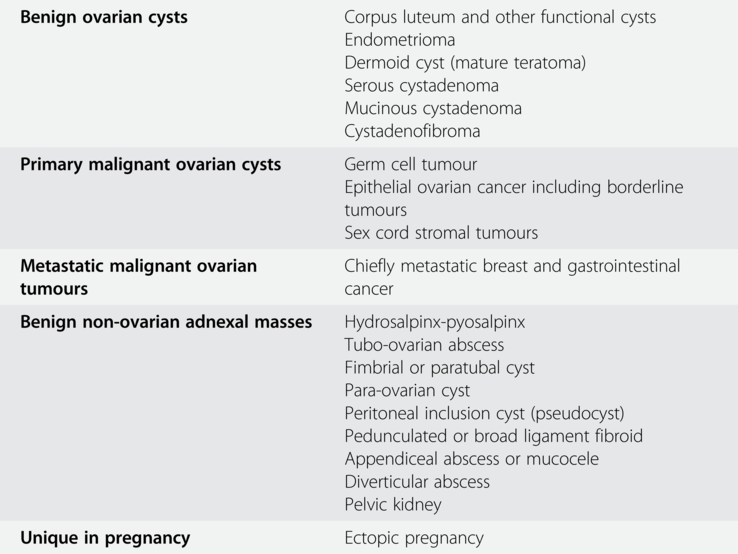
Table 1.1 summarises the most common types of ovarian cysts in pregnancy as well as some non-ovarian adnexal masses, which are essential to consider during differential diagnosis.
Table 1.1 The most common types of ovarian cysts and non-ovarian adnexal masses in pregnancy
| Benign ovarian cysts | Corpus luteum and other functional cysts Endometrioma Dermoid cyst (mature teratoma) Serous cystadenoma Mucinous cystadenoma |
| Primary malignant ovarian cysts | Germ cell tumour Epithelial ovarian cancer including borderline tumours Sex cord stromal tumours |
| Metastatic malignant ovarian tumours | Chiefly metastatic breast and gastrointestinal cancer |
| Benign non-ovarian adnexal masses | Hydrosalpinx-pyosalpinx Tubo-ovarian abscess Fimbrial or paratubal cyst Para-ovarian cyst Peritoneal inclusion cyst (pseudocyst) Pedunculated or broad ligament fibroid Appendiceal abscess or mucocele Diverticular abscess Pelvic kidney |
| Unique in pregnancy | Ectopic pregnancy |
1.3 Clinical Presentation and Complications
Most pregnant women with ovarian cysts are asymptomatic; the cyst is actually an incidental finding either during a routine early pregnancy or antenatal ultrasound examination or during a Caesarean section. Usually, patients become symptomatic either when a cyst enlarges significantly and can be palpated through the abdomen or, more often, if a cyst complication occurs. Physicians should bear in mind that many cyst accident symptoms are non-specific and can easily be mistaken for common pregnancy symptoms (abdominal cramping and discomfort, nausea, vomiting); thus, leading to a delayed diagnosis and management of an acute complication [Reference Bottomley and Bourne5]. Torsion, cyst rupture and haemorrhage are the usual cyst accidents both in non-pregnant and pregnant women. In addition, obstruction of labour by a very large ovarian cyst is a unique to pregnancy, albeit very rare, complication [Reference Alalade and Maraj6].
1.3.1 Torsion
Data on the incidence of ovarian torsion in pregnancy are conflicting, and rates in the literature vary from 0.1 to 15% [Reference Schmeler, Mayo-Smith and Peipert3, Reference Condous, Khalid, Okaro and Bourne4]. While an ovarian torsion may occur even in the absence of a cyst in women with elongated ovarian ligaments, the condition is more common if the ovary is enlarged and ‘heavy’, such as in the presence of large ovarian cysts especially those with solid components (e.g. dermoid cysts) or in ovarian hyperstimulation syndrome. Condous et al. estimated the overall risk of torsion at 0.1%, rising to 5–15% if an ovarian cyst coexists [Reference Condous, Khalid, Okaro and Bourne4]. Approximately 10–20% among all cases of ovarian torsion take place during pregnancy, and 60% of them occur between 10–17 weeks gestation, likely due to cephalad displacement of the ovaries [Reference Huang, Hong and Ding7, Reference Yen, Lin and Murk8]. Large ovarian cysts are also likely to undergo torsion during the early post-partum period; as the uterus involutes, more intra-abdominal space is available for a displaced ovary to undergo torsion.
The symptoms a pregnant woman with ovarian torsion will present with are similar to those of a non-pregnant woman: sudden-onset sharp abdominal pain, constant or intermittent in nature, radiating to the back, groin or flank, nausea, vomiting and occasionally anorexia. On examination, patients may display signs of peritonism, adnexal tenderness and cervical excitation [Reference Bottomley and Bourne5].
Serum markers such as leucocytosis have a limited role in the diagnostic evaluation of pregnant women with a suspected ovarian torsion. On the contrary, transvaginal ultrasonography, with a reported positive predictive value of 87.5% and specificity of 93.3%, is the most useful diagnostic tool [Reference Graif and Itzchak9]. The most common sonographic features observed in ovarian torsion include enlargement of the ovary when compared to the contralateral one, oedema of the ovarian parenchyma and peripherally displaced follicles, perhaps along with transudation of fluid into the displaced follicles. The presence of a displaced ovary (e.g. at the uterine-vesico fold or even at the contralateral side of the pelvis) as well as the ‘whirlpool sign’ on colour Doppler examination (i.e. twisted vascular pedicle) are also useful ultrasound features raising the suspicion of ovarian torsion. Finally, free fluid in the pouch of Douglas can also be noticed in patients with ovarian torsion (as well as in women with ovarian cyst rupture; in the former event, the fluid is reactive in origin and usually its volume is significantly reduced compared to cases of a ruptured cyst).
If ultrasound examination is inconclusive, then MRI can be utilised to investigate a possible ovarian torsion in pregnant women. The use of gadolinium contrast medium should be avoided. On the contrary, CT has no place in the diagnostic work-up of pregnant patients with a suspected ovarian torsion [Reference Senarath, Ades and Nanayakkara10].
It should be flagged that ovarian torsion remains a clinical diagnosis; even though ultrasound imaging is routinely used to aid diagnosis, clinical examination is still the diagnostic cornerstone, and a strong consideration is made to proceed to surgical intervention should the clinical findings be highly suspicious of ovarian torsion.
1.3.2 Ovarian Cyst Rupture and Haemorrhage
Rupture of an ovarian cyst and subsequent haemorrhage, or haemorrhage within an intact ovarian cyst, are quite common cyst accidents that may occur both in pregnant and non-pregnant women. Patients usually present with non-specific symptoms such as sudden-onset lower pelvic pain, nausea and vomiting; of note, pain tends to be worse at the onset of symptoms and may subside by the time of presentation. In the vast majority of cases, the associated haemorrhage is not clinically significant; patients are usually haemodynamically stable; symptoms are manageable with analgesia and tend to resolve within a few days. In the unlikely event of significant intra-abdominal bleeding though, women may present with hypovolemic shock and necessitate an acute surgical intervention.
Similar to ovarian torsion, ultrasound is the first-line imaging modality in suspected ovarian cyst rupture. An experienced sonologist can usually easily identify the presence of haemoperitoneum in the pelvis or even in the upper abdomen and hence, can assess the severity of intra-abdominal bleeding. Furthermore, haemorrhage within an intact ovarian cyst has distinct ultrasound features: a fresh blood clot may display a lace-like or spider-web appearance; it is avascular on Doppler examination and can be seen to ‘wobble’ in a jelly-like fashion on palpation with a transvaginal probe [Reference Bottomley and Bourne5]. The ultrasound appearance of blood may be anechoic in early stages, whereas in later stages accumulated old blood may have a ‘ground-glass’ appearance as in an endometrioma.
1.3.3 Mass Effect: Obstruction of Labour
Like any large pelvic mass, should an ovarian cyst enlarge significantly, it may apply pressure to surrounding structures, including the urinary bladder, ureters, urethra and intestines. The associated symptoms vary and will be determined by which organ is affected and to what degree. The same mechanism is responsible for the obstruction of labour: if a large ovarian cyst is located adjacent to the lower uterine segment and below the presenting part, it may contribute to labour dystocia [Reference Senarath, Ades and Nanayakkara10].
1.4 Diagnostic Evaluation
A thorough medical history and physical examination constitute the mainstay of assessment of pregnant women with ovarian cysts. Further tests such as a full blood count; renal, liver function and electrolytes; C-reactive protein (CRP); coagulation screen; as well as a urine dipstick/culture and triple vaginal swabs may also be useful depending on the clinical scenario, chiefly in acutely unwell patients presenting with suspected cyst accidents [Reference Bottomley and Bourne5]. The diagnostic work-up would be incomplete without the use of imaging modalities to characterise the nature of the cyst, to discriminate between benign and malignant lesions, to investigate any possible acute cyst complications and to guide further management.
1.4.1 Imaging
Ultrasonography is considered the first-line imaging modality in pregnant women with an ovarian cyst [Reference Eskander, Berman and Keder11]. It is safe both for the mother and the fetus, widely available and cheaper compared to alternative options; hence, it is an ideal primary evaluation tool.
Transvaginal ultrasound can be used as early as the fourth to fifth week of gestation to confirm the implantation site and exclude an extrauterine or heterotopic pregnancy. Assessment of the ovaries and the Fallopian tubes is becoming standard practice during an early pregnancy scan, allowing early identification of adnexal cysts, even if they are quite small and asymptomatic.
Accurate characterisation of the nature of ovarian cysts identified in pregnancy is crucial. Subjective pattern recognition (i.e. subjective impression of the sonologist) has a very good diagnostic performance for the assessment of adnexal cysts with a sensitivity and specificity equal to those of complex logistic regression models [Reference Sokalska, Timmerman and Testa12, Reference Kaloo, Louden, Khazali and Hoy13]. However, this requires an expert ultrasound operator, and it remains questionable whether it can be safely applied to a non-expert setting [Reference Yazbek, Raju and Ben-Nagi14]. As a result, many algorithms and models have been developed in an attempt to improve the diagnostic accuracy of non-expert examiners. Some of these models, such as the Risk of Malignancy Index (RMI), take into account other factors besides the ultrasound characteristics (e.g. cancer antigen-125 (CA-125)), some of which alter during pregnancy and hence, cannot be applied to pregnant women [Reference Kaloo, Louden, Khazali and Hoy13].
The International Ovarian Tumor Analysis (IOTA) group has published extensively on ultrasound-based models to differentiate between malignant and benign ovarian cysts. The group has proposed ‘simple descriptors’ and ‘simple rules’. These help non-expert operators classify most ovarian lesions as benign or malignant with a reported sensitivity of 95% and specificity of 91%, leaving only a small proportion of indeterminate cases to be assessed by expert sonologists [Reference Timmerman, Testa and Bourne15, Reference Ameye, Timmerman and Valentin16]. Table 1.2 presents the IOTA ‘simple rules’ and ‘simple descriptors’ for differentiation between malignant and benign adnexal lesions [Reference Timmerman, Testa and Bourne15].
Table 1.2 The IOTA ‘simple rules’ and ‘simple descriptors’ for differentiation between malignant and benign adnexal lesions
The pregnant status of patients should always be kept in mind during an ultrasound evaluation of an ovarian cyst. Even benign cysts such as endometriomas may undergo major morphological changes during pregnancy, referred to as decidualisation, and mimic malignancy. The effect of pregnancy-related high progesterone levels on the ectopic endometrial tissue of an endometrioma results in the formation of ectopic decidua. Sonographically, a decidualised endometrioma is characterised by a thick, irregular inner wall and prominent intraluminal papillary projections with increased blood flow on Doppler examination, similar to malignant ovarian tumours. As shown by Pateman et al. [Reference Pateman, Moro and Mavrelos17], about 12% of endometriomas will undergo decidualisation during pregnancy. In such cases, ultrasound examiners should look for other features to confirm their benign nature, such as the presence of extraovarian nodules of deep infiltrating endometriosis (e.g. in the uterosacral ligaments, the rectovaginal space or the rectosigmoid colon), obliteration of the pouch of Douglas as well as the tendency of endometriomas to decrease in size and regress rapidly during pregnancy, contrary to borderline and malignant lesions [Reference Pateman, Moro and Mavrelos17].
A proportion of pregnant women with an ovarian cyst will require further imaging tests. In such cases, MRI is the test of choice. Contrary to ultrasonography, MRI is more operator-independent; moreover, it has excellent resolution for soft-tissue pathology, high sensitivity in identifying malignancy and negligible risks to the mother and fetus [Reference Adusumilli, Hussain and Caoili18]. The use of gadolinium contrast medium should be avoided due to its teratogenic effect; actually, it is usually not required for the assessment of ovarian pathology [Reference Ball, Waters and Cooper19]. Left lateral positioning should be considered to avoid caval compression by the gravid uterus in case of a prolonged stay in an MRI scan machine.
The use of CT for the assessment of ovarian cysts in pregnant women is not recommended; besides the well-established safety concerns about ionising radiation, CT actually performs poorly when compared to ultrasonography and therefore, it does not offer any clinical benefits [Reference Senarath, Ades and Nanayakkara10].
1.4.2 Tumour Markers
Contrary to non-pregnant women, the use of serum tumour markers in patients with ovarian cysts during pregnancy is limited. The physiology of pregnancy alters baseline levels of some of these markers; the interpretation of abnormal results should be made with caution and always in conjunction with the results of imaging tests.
CA-125 is raised in about 80% of women with epithelial ovarian cancer, and it is the most frequently used serum tumour marker even though its specificity is low [Reference Kaloo, Louden, Khazali and Hoy13]. Its baseline levels increase in pregnancy; they peak in the first trimester with the upper limit of the normal CA-125 range reaching 112 U/mL between 11 and 14 weeks gestation and then decreasing as gestation advances [Reference Aslam, Ong, Woelfer, Nicolaides and Jurkovic20]. Even though measuring CA-125 values per se will not discriminate between benign and malignant lesions in pregnancy, it may have some merits in suspicious or indeterminate ovarian cysts, as a significantly raised level may flag a potential malignancy and trigger further investigations. It may be measured as a reference point before and after treatment in women with known ovarian cancer, thus allowing physicians to monitor the response to treatment [Reference Alalade and Maraj6].
Other serum tumour markers used to monitor germ cell tumours include alpha-fetoprotein, human chorionic gonadotropin and lactate dehydrogenase. Unfortunately, the former two increase physiologically during pregnancy and therefore are of limited use. The latter is not affected by pregnancy, and its raised levels are a significant marker, commonly noted in dysgerminoma [Reference Alalade and Maraj6].
Finally, the glycoprotein HE4, which is a more sensitive and specific marker than CA-125 in differentiating between benign and malignant ovarian tumours, has lower baseline levels during pregnancy, hence limiting its potential use in pregnant women with ovarian lesions [Reference Alalade and Maraj6].
1.5 Management
Management of ovarian cysts diagnosed during pregnancy is challenging; physicians must address the individual needs of two patients, the mother and the fetus. Several factors need to be considered during decision-making: patient symptoms, gestational age and fetal well-being at the time of presentation, likelihood of malignancy or cyst accident, maternal and fetal risks associated with each management option, nature of surgical intervention and appropriate surgical approach. Each case needs to be assessed individually, ideally by a multidisciplinary team (MDT) comprising one or more obstetricians, gynaecologists, imaging specialists, paediatricians and midwives. In case a borderline or malignant ovarian lesion is suspected, then the case should also be referred to and managed by the gynaecology–oncology MDT including gynaecology oncologists, medical oncologists, radiologists, clinical nurse specialists and psychologists. This approach is likely to benefit the patient with a range of opinions and expertise collaborating in the management plan.
In general, the approach may include expectant management, ultrasound-guided fine needle aspiration or surgical management with the main determinants being patient symptoms and whether imaging findings are reassuring or raise any suspicions about malignancy.
1.5.1 Expectant Management
This is a safe approach in asymptomatic women with benign looking ovarian cysts without any suspicious imaging features; it is also supported by a recent evidence-based guideline commissioned by the British Society for Gynaecological Endoscopy (BSGE) and endorsed by the Royal College of Obstetricians and Gynaecologists (RCOG) [Reference Ball, Waters and Cooper19]. The vast majority of cysts diagnosed in pregnancy are benign and actually, most of them are functional in nature. About 70% of them will resolve spontaneously by 16 weeks gestation and hence, no interventions are needed [Reference Condous, Khalid, Okaro and Bourne4].
It has been suggested that cysts with benign features measuring more than 6 cm in size or those with a complex, albeit reassuring, ultrasound appearance should be followed up after four to six weeks [Reference Alalade and Maraj6, Reference Senarath, Ades and Nanayakkara10]. This is a reasonable practice but nevertheless, an experienced sonologist with expertise in women’s imaging can, most of the time, offer reassurance about the scan findings and reduce the need for routine serial scans. Of course, certain types of benign ovarian cysts such as endometriomas should be followed up during pregnancy given the decidualisation-related diagnostic challenges.
When expectant management is favoured, women should be counselled about the risk of ovarian torsion and cyst rupture, and the plan should be reviewed if a patient becomes symptomatic and a cyst accident is suspected.
1.5.2 Ultrasound-Guided Fine Needle Aspiration
This option applies only to symptomatic women with simple benign ovarian cysts. It is a straight-forward outpatient procedure performed under local anaesthesia and it can provide immediate symptomatic relief, therefore reducing the need for admission and more invasive procedures. It is also included in the recent BSGE–RCOG guideline as an alternative option to surgery [Reference Ball, Waters and Cooper19]. However, some authors have expressed concerns about high recurrence rates (33–40%), as well as the potential risk of intraperitoneal spillage of cancerous cells in the case of an undetected malignancy [Reference Bottomley and Bourne5, Reference Guariglia, Conte, Are and Rosati21].
1.5.3 Surgical Management
Surgery is usually reserved for patients with an acute abdomen due to a cyst accident and women with imaging findings suspicious of malignancy. It should also be considered in asymptomatic patients with large ovarian cysts (usually more than 10 cm, although there is no consensus in the literature about a cut-off limit) to prevent complications such as torsion, rupture or obstruction of labour [Reference Alalade and Maraj6].
Historically, the surgical approach included a laparotomy, nevertheless advances in minimal access surgery have now established laparoscopy as a safe and feasible approach during pregnancy [Reference Ball, Waters and Cooper19]. A growing volume of literature evidence has demonstrated its superior outcomes in patients with benign disease and having addressed previous safety concerns, recent guidelines reflect the change in practice [Reference Ball, Waters and Cooper19]. Laparoscopic surgery offers the same advantages to pregnant as to non-pregnant women. High quality studies have confirmed that laparoscopy is associated with improved visualisation of pelvic organs, reduced blood loss, less pain, reduced length of hospitalisation, faster recovery and a lower risk of uterine irritability compared to laparotomy, without an increase in adverse obstetric outcomes (miscarriage, preterm delivery or fetal growth restriction) [Reference Chen, Ding and Hua22, Reference Liu, Zhang, Huang and Wang23]. Patients mobilise more quickly following a laparoscopy, which is important given the hypercoagulable pregnancy status.
Previous concerns with regard to the impact of pneumoperitoneum and carbon dioxide on uteroplacental flow have now been discarded [Reference Candiani, Maddalena and Barbieri24]. A study by Reedy et al. did not find any statistically significant differences in five fetal outcome variables (birth weight, gestational duration, growth restriction, infant survival and fetal malformations) in women with singleton pregnancies between 4–20 weeks of gestation undergoing laparoscopy versus laparotomy [Reference Reedy, Källén and Kuehl25]. Most literature data as well as the BSUG–RCOG guidelines recommend an intra-abdominal operating pressure of 12 mmHg to minimise the risk of alterations to the feto-maternal perfusion, although visualisation may become more challenging [Reference Ball, Waters and Cooper19, Reference Candiani, Maddalena and Barbieri24]. An operating pressure of 15 mmHg, however, has been used without adverse fetal or maternal outcomes [Reference Chen, Ding and Hua22, Reference Pearl, Price, Tonkin, Richardson and Stefanidis26].
Another potential concern is related to the risk of inadvertent damage to the pregnant uterus with the Veress needle or the primary port during entry into the abdominal cavity. No randomised controlled trials have compared the safety of various laparoscopic entry points and techniques during pregnancy, therefore operating surgeons may utilise alternative options (e.g. open Hasson technique or direct gasless entry with optical trocar, entry at Palmer’s point, supra-umbilical or sub-xiphoid point) according to their preference, fundal height and location of the ovarian cyst [Reference Ball, Waters and Cooper19].
Despite its advantages, laparoscopic surgery during pregnancy can be technically challenging especially after the first trimester due to the enlarged pregnant uterus, absence of intra-uterine manipulation, relatively low intra-abdominal pressures and reduced intra-abdominal space. Therefore, it is recommended that that such procedures should be performed by experienced laparoscopic surgeons with advanced skills [Reference Ball, Waters and Cooper19]. If malignancy is suspected pre- or intra-operatively, then a laparotomy should be considered to reduce the likelihood of cyst rupture and spillage of cancerous cells.
1.5.3.1 Elective versus Emergency Surgery
A recent systematic review and meta-analysis by Cagino et al. revealed that elective surgery during pregnancy is associated with a lower risk of preterm birth compared to emergency surgery [Reference Cagino, Li and Thomas27]. As highlighted above, each case should be individually assessed within an MDT-based environment. Nevertheless, it seems that in the absence of any other robust data to guide counselling, elective surgery of asymptomatic, particularly large ovarian cysts should be considered to prevent complications and improve outcomes.
1.5.3.2 Cystectomy versus Oophorectomy
The decision on the nature of the surgical intervention (cystectomy versus oophorectomy or more radical surgery) depends on preoperative imaging and intra-operative surgical findings. Should there be no suspicion of malignancy, then a cystectomy is the approach of choice if feasible, with the aim to preserve as much healthy ovarian tissue as possible. Efforts should be made to avoid peritoneal spillage of cyst contents to prevent chemical peritonitis in the case of dermoid cysts and dissemination of malignant cells in the case of an undetected malignancy. The use of tissue removal bags or even in-bag dissection is encouraged. The corpus luteum should not be damaged if possible during surgical interventions in the first trimester. Drainage of benign looking ovarian cysts with or without concomitant cystectomy is an acceptable and safe alternative during pregnancy [Reference Ball, Waters and Cooper19].
If a borderline or malignant ovarian lesion is suspected, then a two-stage approach could be considered especially in presumed early-stage disease; primary surgery during pregnancy may include a unilateral salpingo-oophorectomy and surgical staging (cytology, peritoneal biopsies, omentectomy and appendicectomy) with a completion staging surgery performed after delivery [Reference Mukhopadhyay, Shinde and Naik28]. Of course, the gynaecology–oncology MDT will guide surgical planning of such cases.
1.5.3.3 When to Operate?
Historically, the second trimester was thought to be the ideal time to perform elective surgery in pregnancy, minimising the first and the third trimester risks of miscarriage and preterm birth, respectively. Nevertheless, this was not based on high quality evidence. A growing amount of literature data have demonstrated that laparoscopic surgery can be safely performed during all trimesters of pregnancy without any additional maternal and neonatal risks [Reference Reedy, Källén and Kuehl25, Reference Weiner, Mizrachi and Keidar29]; recent evidence-based guidelines from the UK and US societies of endoscopic surgeons reflect this change in practice [Reference Ball, Waters and Cooper19, Reference Pearl, Price, Tonkin, Richardson and Stefanidis26].
Excision of an ovarian cyst at the time of a Caesarean section can be considered, especially if previous imaging has established that it is not a functional cyst or in the case of unexpected suspicious features. In general, management of such cases should be individualised and senior involvement should be sought in the case of an incidental finding of an ovarian cyst during a Caesarean section.
1.5.3.4 Obstetrical Considerations and Fetal Monitoring
Pre- and post-operative fetal heart monitoring is recommended to confirm fetal well-being. Contrary to past practice, routine intra-operative monitoring is no longer deemed necessary, as fetal heart and maternal uterine artery Doppler studies during laparoscopic surgery did not reveal any abnormalities [Reference Ball, Waters and Cooper19, Reference Candiani, Maddalena and Barbieri24, Reference Pearl, Price, Tonkin, Richardson and Stefanidis26].
Women undergoing surgery between 24 ± 0 and 35 ± 6 weeks gestation with a risk of preterm birth should be administrated antenatal corticosteroids for lung maturity; magnesium sulphate should also be used up 33 ± 6 weeks gestation for fetal neuroprotection as per the National Institute for Health and Care Excellence (NICE) guidelines [30].
Prophylactic administration of tocolytics has not been found to improve outcomes and hence, it is not routinely recommended; of course, should there be signs of preterm birth, this can be considered [Reference Ball, Waters and Cooper19, Reference Pearl, Price, Tonkin, Richardson and Stefanidis26].
1.6 Post-Natal Follow-Up
Post-natal ultrasound evaluation of persistent ovarian cysts, usually six weeks post-partum, is a reasonable approach. At this stage, patients will be able to undergo elective surgery if deemed necessary [Reference Senarath, Ades and Nanayakkara10].
1.7 Conclusion
Since ultrasonography was established as an integral part of antenatal care, the incidence of diagnosis of ovarian cysts in pregnant women has increased. The vast majority of them are functional in nature; such cysts can be safely managed expectantly. The main objective of assessment and management is to exclude possible acute cyst accidents, triage and fast-track cysts with suspicious features and optimise maternal and fetal outcomes; however, this can often be challenging due to the pregnant state of patients masking pathology or confounding symptoms. Shared decision-making within an MDT-based environment is encouraged.
Clinical Governance Issues
Most ovarian cysts encountered in pregnancy are functional in nature and will resolve spontaneously by 16 weeks gestation
Accurate characterisation of the nature of the cysts is essential. Approximately 1–5% and 1–2% of them will represent a malignant (most commonly germ cell, sex cord stromal and epithelial tumours) and a borderline lesion (predominantly serous and mucinous borderline tumours), respectively
The possibility of a cyst accident (e.g. ovarian torsion, cyst rupture and haemorrhage) should always be considered in pregnant women presenting with lower abdominal pain
About 10–20% of all cases of ovarian torsion occur during pregnancy, usually between 10–17 weeks gestation
Although ovarian torsion has distinct ultrasound features, it still remains a clinical diagnosis. In the absence of convincing imaging features, physicians should strongly consider proceeding to surgical intervention should the clinical findings be highly suspicious of ovarian torsion
Ultrasonography is the first-line imaging modality in pregnant women with an ovarian cyst
The IOTA group’s ‘simple descriptors’ and ‘simple rules’ have high sensitivity and specificity in differentiating between benign and malignant ovarian cysts
Endometriomas may undergo major sonographic changes during pregnancy (decidualisation) and mimic malignancy
Physiology of pregnancy alters baseline levels of most tumour markers, hence their role during pregnancy is limited
MRI can be a useful diagnostic tool should ultrasonography be inconclusive, as it has excellent resolution for soft-tissue pathology, high sensitivity in identifying malignancy and negligible risks to mother and fetus
Pregnant women with an ovarian cyst should be managed within an MDT-based environment
Expectant management is a safe option in asymptomatic women with benign looking ovarian cysts
Ultrasound-guided fine needle aspiration is a reasonable alternative in symptomatic women with simple benign ovarian cysts despite the high recurrence rates
Surgery should be considered in patients with acute abdomen, women with suspected malignancy and possibly in asymptomatic patients with large ovarian cysts to prevent future complications
Laparoscopic surgery in all trimesters of pregnancy is feasible and safe both for the mother and fetus. It should be performed by experienced surgeons with advanced laparoscopic skills especially at advanced gestational age