General principles
Patients on ECMO must be managed in an intensive care environment. In addition to the specific elements monitored in the circuit (see Chapter 3), standard ICU continuous monitoring is routinely used.
Bedside observations by a trained nurse are vital. These observations should encompass both patient and circuit. Continuous awareness of the potential issues is important, as a rapid response to events is required to avoid catastrophic consequences.
Standard monitoring will include, but not be restricted to, continuous cardiac rhythm monitoring, pulse oximetry, invasive arterial and venous blood pressures, temperature, respiratory rate and end-tidal CO2. These will be documented at regular intervals. Hourly observations will include fluid intake and output, and the overall fluid balance will be calculated. Circuit data will also be documented.
Intravascular pressure can be measured in the same way as in non-ECMO patients. Of note, any indwelling venous catheter is a potential source of air that could be entrained into the ECMO circuit due to the high negative pressure. The position of the cannula (see Chapter 6) in relation to the pressure monitoring may affect the pressure being read. The pressure could be falsely elevated if the cannula and tip of the indwelling catheter are close to each other, or too low if influenced by the negative pressure next to a drainage cannula.
Many invasive monitoring devices (used mainly to assess cardiac output and derive parameters such as extra lung water) have not been validated in ECMO patients and should only be used with extreme caution. These devices have usually not been validated in extreme conditions, and ECMO is not only extreme but introduces a new physiological dimension (which could be called a new physiological bed).
Arterial blood gas analysis will be required at regular intervals and when clinically indicated.
X-rays (chest or abdomen) are useful tools in assessing cannula position and lung recovery (or damage). Ultrasonography, mainly echocardiography, is useful but can only be used intermittently.
Neuromonitoring can be used but interpretation might be difficult. The recorded signals will be affected by O2 blood saturation and regional blood flow regulation. ECMO and drugs will affect regional blood flow distribution, and it is likely that this will change the way the signal is interpreted. Specific devices, such as near-infrared spectroscopy, only measure what happens in a very small area of the brain, and generalization of the reading is not possible. Moreover, normal ranges are not defined. Transcranial Dopplers can be used, but it is unclear how they affect management and outcome.
All standard principles will apply, but the clinician has to integrate the extracorporeal gas exchange in the interpretation of all changes. Specifics to veno-venous or veno-arterial ECMO are described below.
Distal arterial perfusion and venous drainage can be affected by ECMO cannulas. Most common is the lack of perfusion of the limb distal to the insertion of an arterial cannula, and hence the use of reperfusion lines (see Chapter 6). Oedema can occur if venous drainage is impaired. Careful observation is required, as the consequences can be devastating (loss of a limb, or even cerebral oedema when the flow through the internal jugular is impaired).
Monitoring the patient on veno-venous ECMO
In veno-venous ECMO, the blood is taken from and returned into the venous circulation. The end result is a venous blood with higher O2 and lower CO2 content entering the pulmonary circulation. The blood will mix with the patient’s blood that is not going through the ECMO circuit. The final content of the venous blood entering the heart will be based on the admixture of both ECMO and native circulation.
This means that a higher cardiac output will increase the proportion of patient’s native (non-EMCO) blood in the final mix; while higher ECMO flow will increase the proportion of ‘ECMOed’ blood in the final mix. This explains in part why higher ECMO flow brings higher oxygenation!
A final mix with higher O2 content will affect hypoxic vasoconstriction (but the extent of this, in the context of critically ill patients receiving multiple drugs is unknown).
Oxygen content will be a result of both the blood returned by the ECMO circuit and the patient’s own vascular beds.
Changes in ventilation and perfusion will affect the end-tidal CO2.
When on veno-venous ECMO, most of the monitoring will be directed to observing the recovery of the lungs to ensure that they are not further damaged.
The suction of venous blood is likely to affect any system derived on thermodilution and caution should be exerted when using techniques relying on a venous injectate. Observing the distribution of the contrast dye injected during a CT scan with contrast clearly illustrates that an unknown and variable quantity of diluent is sucked into the ECMO circuit (and returned with some delay).
In veno-venous ECMO, one of the key issues is recirculation of oxygenated blood around the ECMO circuit itself. This happens when the drainage and return cannula are too close to each other, or are located in such a way that the oxygenated blood will be preferentially returned to the ECMO circuit rather than the right heart circulation. The efficiency of the ECMO will decrease substantially as the blood will be saturated in O2 and low in CO2, and the ECMO will in effect support the ECMO. Changes in the patient physiology during support may increase or decrease the amount of blood being recirculated. Dramatic changes can be seen when observing the colour of the blood in the circuit but more subtle ones require the PaO2 to be measured in the circuit before the oxygenator. This value will however vary according to the patient’s own O2 extraction and other physiological changes, and is therefore sometimes difficult to detect. Confirmation of recirculation will often require careful mobilization of the cannula. Recirculation is illustrated in Figures 4.1 and 4.2.

Figure 4.1 Illustration of recirculation in a patient on veno-venous ECMO. The oxygenated blood returned to the patient is immediately aspirated by the ECMO circuit.

Figure 4.2 In a system with two drainage cannulas, it is obvious that the blood in one drainage tubing has the same colour as the blood returned to the patient, indicating recirculation.
Monitoring the patient on veno-arterial ECMO
In veno-arterial ECMO, the blood is taken from the venous circulation and returned to the arterial circulation. The end result is arterial blood with a higher O2 and lower CO2 content entering the systemic circulation.
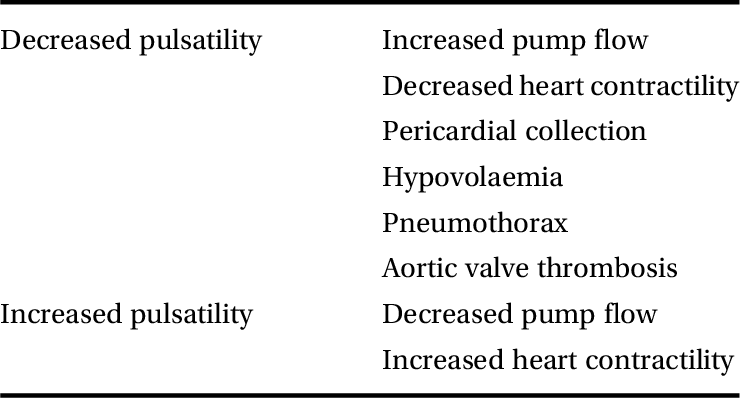
Veno-arterial ECMO bypasses the cardiopulmonary circulation, and the risk of no flow in the pulmonary vessels is high. This may lead to thrombosis. In the absence of left ventricular ejection (which often occurs if the heart is weakened and its afterload is increased by the pressure generated by the ECMO), ventricular cavities will distend and thrombi are likely to be formed. It is therefore important to ensure that there is continuous blood flow through the lungs and no stagnation of blood in the cardiac cavities. Ensuring opening of the aortic valve is required, and this can usually be observed on the pressure waveform. Using a pulmonary artery catheter allows continuous monitoring of pulmonary blood flow. Changes in the pressure waveforms must be recorded and discussed, as they will reflect a change in one of many aspects in the patient’s care. The reasons for changes in the arterial pressure waves are listed in Table 4.1.
Table 4.1 Possible reasons for changes in arterial pressure waveform in a patient on veno-arterial ECMO
| Decreased pulsatility | Increased pump flow |
| Decreased heart contractility | |
| Pericardial collection | |
| Hypovolaemia | |
| Pneumothorax | |
| Aortic valve thrombosis | |
| Increased pulsatility | Decreased pump flow |
| Increased heart contractility |
If the patient is still pumping blood through the native circulation, the blood exiting the cardiopulmonary circulation (‘native’ blood) will mix with that pumped through the ECMO circuit. The ‘native’ blood will be more or less oxygenated, and CO2 will have been more or less cleared, depending on the conditions of the lungs. Both the ECMO and the ‘native’ blood will meet and mix, but this will be affected by many physiological variables.
If the patient’s cardiac output is increasing but the lungs are not functioning properly, the proportion of poorly oxygenated blood will increase (assuming the ECMO flow remains the same, although this will usually not be the case, as a better cardiac function will increase systemic pressure and this in turn will decrease ECMO flow if the pump energy is not increased). The flow distribution is such that some areas may receive hypoxic blood, while others may be hyperoxic. A patient can appear to be very well oxygenated (i.e. be pink and have a high measured PaO2) but have an ischaemic electrocardiogram (ECG). This will happen when the blood exiting the cardiopulmonary circulation and entering the coronary arteries has not been properly oxygenated, while the very well oxygenated ECMO blood is distributed in all other vascular beds except the coronaries. A patient can appear bicoloured (called harlequin syndrome) when part of the circulation is supplied by poorly oxygenated ‘native’ blood and the rest by ECMO blood. This will happen, for example, in patients with peripheral veno-arterial ECMO where the blood is returned in the femoral artery. The blood from the ECMO circulates in the lower portion of the body, while the blood pumped by the heart circulates in the upper portion. On some occasions, a demarcation line can be observed. Arterial blood gases taken at various locations will give values of PaO2 that can be very different. Of note, interrupting the sweep gas in a veno-arterial circuit (something that should NEVER be done) will inject blood with a low O2 content and a reverse harlequin effect could be seen.
In veno-arterial ECMO, venous O2 content will be related to O2 extraction (itself affected by O2 content in the arterial circulation). Venous saturation can be monitored, but the clinician has to understand that this value will be affected by many variables (mixed venous oxygen saturation (SvO2) is always affected by many variables, even in non-EMCO patients) and must be interpreted with caution.
Bypassing the cardiopulmonary circulation will affect pulmonary blood flow. This will affect pulmonary artery pressures. Similarly, the end-tidal CO2 will be affected by the quantity of blood entering the lung and where it flows (areas that are ventilated or not). The pulmonary capillary wedge pressure can be measured, although great caution is advised in the anticoagulated patient. The variation in pulmonary and intracardiac blood flow is likely to impact on the validity of the measured pressures, but trends can be useful.
In veno-arterial ECMO, there is no recirculation of oxygenated blood in the ECMO circuit itself.
Echocardiography is useful, although it only provides intermittent monitoring of cardiac recovery. Repeated transoeosophageal examination can lead to trauma, and transthoracic examination is preferred. While many indices can be measured, the most useful are still a subjective assessment of function and visualization of valve opening.
Measuring non-invasive pressures may not be possible in the patient with veno-arterial ECMO and with absence of pulsatility. In these cases, sphygmomanometry and a Doppler to detect blood flow are used.
Key points
Patient monitoring is at least the same as in all patients in the ICU.
Veno-arterial ECMO impact on standard monitored values is highly complex.
There is no reliable way to measure cardiac output while on ECMO.
Neuromonitoring is not standardized for the ECMO patient.