Haematology input
Expert haematology support is required when supporting patients on ECMO.
Extracorporeal circulation is only possible if the patient’s coagulation is controlled. Exposure of blood to foreign materials triggers the inflammatory response, including the coagulation cascade. Protocols and guidelines for staff must be in place to help manage a fine balance, minimizing both the risk of thrombus formation and that of spontaneous (or aggravated) bleeding.
The blood passing through the circuit will be exposed to different forms of stresses. These will add to the modifications caused by the primary disease or clinical problem leading to the patient requiring support.
Bleeding justifies transfusion of replacement products.
Patients can present with primary haematological disease compounding the complexity of their management.
Most patients will be adequately managed by experienced staff referring to guidelines, but expert advice should always be sought for any deviation.
General considerations
The need for anticoagulation
Most patients supported by ECMO will receive continuous anticoagulation. In some circumstances, the risk of anticoagulation will outweigh its potential benefits.
Heparin-coated circuits allow avoidance of systemic anticoagulation as long as the blood flow is maintained at a high enough rate (probably above 2 L/min) in each component of the circuit. While this seems to be possible for weeks in patients supported with veno-venous ECMO, we would not advocate it in patients requiring veno-arterial ECMO. The changes in flow rheology observed during veno-arterial ECMO may explain why thrombi rapidly develop in blood vessels or cardiac chambers.
Patients supported with ECMO are at risk of dramatic blood loss. These can be iatrogenic (e.g. surgical wound) or spontaneous (e.g. epistaxis or retroperitoneal haemorrhage). Anticoagulant drugs must be easy to titrate and to reverse.
Thrombi formed in a veno-venous circuit that reach the venous circulation will be filtered by the lungs. Thrombi formed in veno-arterial circuits that reach the systemic circulation will have immediate dramatic consequences.
Assays and blood samples
The majority of assays are done using plasma, as whole blood is unstable. The majority of coagulation assays are based on platelet-poor plasma, usually requiring a 10 min centrifugation step. This explains the time-delay between request and result, and the frustration sometimes encountered by the clinical team looking after critically ill patients.
The majority of laboratory analysers are optical instruments. Extreme and sudden changes in the patient’s whole-blood composition will impair the laboratory’s ability to obtain and interpret valid results. This applies to patients presenting with lipaemia or haemolysis.
To analyse coagulation in particular, mechanical instruments such as the old-fashioned steel ball coagulometer or thromboelastograph can get results where optical instruments will fail.
Frequent blood sampling is a significant source of iatrogenic anaemia. Intelligent scheduling and the use of smaller quantities (such as used in paediatric patients) may help to alleviate this issue.
Choice of anticoagulant
The ideal anticoagulant for patients on ECMO would be highly effective in reducing thrombotic events; carry a low rate of bleeding events; exhibit a rapid and predictable dose response in a variety of clinical scenarios; have easy, cheap and accurate point-of-care monitoring; and have no drug interactions.
Unfractionated heparin
Unfractionated heparin remains the mainstay of continuous anticoagulation therapy in the patient on ECMO support due to its rapid onset of action and the ability to reverse its action with protamine. It is used daily in thousands of patients.
When given intravenously, the heparin half-life is short (30–90 min depending on dose). The onset is immediate when given as a bolus. Heparin can be used to form an inner anticoagulant surface on various devices, such as ECMO tubing, cannulas and oxygenator components.
Pharmacological heparin is a complex bioextract obtained from bovine lung or porcine intestine. The anticoagulant activity per unit of heparin varies among manufacturers, as does its reversibility by protamine in U/mg.
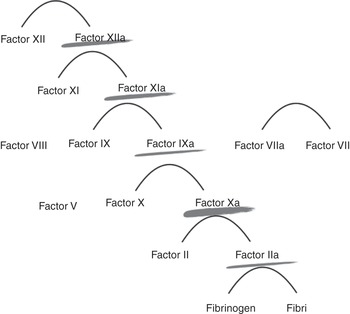
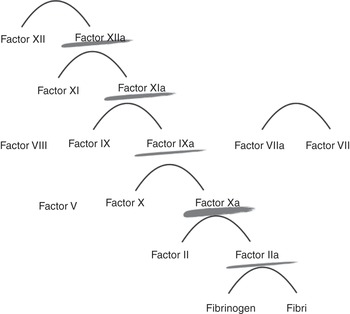
Heparin action is dependent on the presence of functional antithrombin in the patient and acts on the clotting factors IIa, Xa, IXa and XIa. The avidity to these factors will vary depending on the unfractionated heparin chain length and sulfation.
Elimination is both by poorly characterized internal absorption pathways (through macrophages, platelets and endothelium) and renally. Clinically significant half-life prolongations are not observed in renal failure.
The optimal dose of heparin sufficient to prevent thrombosis without causing bleeding is not known.
Fractionated heparin and pentasaccharides
Drugs such as low-molecular-weight heparin (LMWH) and fondaparinux are not used routinely in patients supported with ECMO as they cannot easily be reversed and have long half-lives in some circumstances. New antidotes are being developed and may allow the safe use of agents other than heparin in patients supported with ECMO.
Fractionated heparin or LMWH are the result of breaking down polymeric heparin salts into smaller components. Their effect is easier to predict than those of unfractionated heparin. Their action is dependent on antithrombin. Despite being a shorter version of heparin, LMWH has a long half-life and is excreted exclusively by the kidneys. The reversibility varies, and LMWH can be partially reversed by protamine. This is more effective shortly after the LMWH has been administered but cannot entirely be predicted, so is impractical.
Fondaparinux is a pentasaccharide and its action is antithrombin dependent. Fondaparinux cannot be reversed with protamine and has a very long half-life (17 h). It is excreted by the kidneys.
Monitoring of anticoagulation
The effect of anticoagulants has to be monitored closely to achieve the best balance between avoidance of thrombus formation and risk of bleeding. This can usually be achieved with heparin levels between 0.3 and 1.0 U/mL. Of note, this is around five times lower than the concentration obtained during cardiac surgery with cardiopulmonary bypass (during which the blood is subjected to surgical trauma and exposed to air).
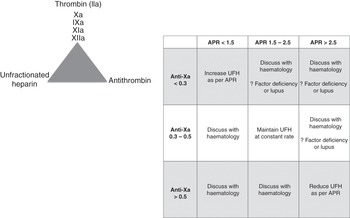
Heparin acts at various levels of the coagulation cascade, as shown in Figure 7.1.
Heparin monitoring
There is no calibrated reference standard, as seen for anticoagulation with vitamin K inhibitors for which the international normalized ratio (INR) is used. The anti-factor Xa (anti-Xa) level is the closest reference standard available.
Activated coagulation time
The activated coagulation time (ACT) was devised by Hattersley in 1966 for the care of patients with severe haemophilia requiring heparin monitoring. The ACT records the time between exposure in a tube of whole blood to glass (contact activation) and the formation of a visible thrombus.
The ACT is sensitive to heparin and measures the heparin effect rather than the level. Factors beyond the plasma coagulation affect the absolute result, as it is a whole-blood assay.
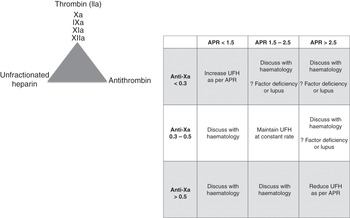
There is no universally agreed therapeutic range. It is a near-patient assay. Most hospitals have not validated their in-house ACT cartridges and do not know how this value correlates with their coagulation tests (which are highly variable from institution to institution). Each hospital should validate the ACT measurements to either their laboratory activated prothrombin time (aPTT) or anti-Xa measures to ensure that the adopted ACT range is at least comparable to the laboratory coagulation tests range. An example is shown in Figure 7.2. The ACT tests will vary in their sensitivity to heparin, and different systems should be used to monitor various clinical settings (e.g. an ACT system designed to be used during cardiac surgery will monitor much higher doses of heparin than systems used during ECMO).
Activated prothrombin time
The aPTT is triggered by exposing platelet-poor plasma to phospholipids and sand. Centrifugation from whole blood to platelet-poor plasma introduces a delay of 10 min in obtaining a result. Using platelet-poor plasma introduces a bias that is dependent on the composition of the patient’s whole blood when trying to compare it directly with the ACT. The aPTT is relatively inexpensive.
The aPTT measures the heparin effect rather than the heparin level. It can be validated against heparin protamine titration by performing the test repeatedly with increasing doses of protamine added to neutralize the heparin effect in the sample.
The activated prothrombin time ratio (APR) is a modification of the aPTT result: the patient’s aPTT (measured in seconds) is divided by the mean of the normal range (in seconds). The APR is a dimensionless ratio (e.g. 1.5) but must not be confused with the INR. The aPTT range and APR must be standardized to heparin protamine titration in each laboratory, as it is dependent on the reagent providers or analysers.
The sensitivity of the test varies between different reagent manufacturers. Care must be taken when adopting the aPTT ranges validated in another centre, as this may use a different aPTT/analyser combination.
The aPTT will also be affected by the presence of lupus anticoagulant, or by changes in factor VIII, IX, XI and XII levels.
Anti-Xa levels
The anti-Xa level has replaced heparin protamine titration as the ‘gold standard’ test for some of the fractionated heparins.
The anti-Xa level can measure heparin effect (if patient endogenous antithrombin is used in the assay) or heparin level (if exogenous antithrombin is added to the assay). The ECMO physician should establish whether the assay uses exogenous antithrombin or not, as this will affect interpretation of the results.
The anti-Xa level measurement is performed in platelet-poor plasma and therefore is not comparable to the whole-blood ACT. The anti-Xa level does not measure the inhibition of factors XIa, IXa and IIa, and will only reflect part of the unfractionated heparin activity.
The anti-XA level will only be slightly affected by global changes in coagulability, such as that observed in disseminated intravascular coagulation.
The anti-Xa level requires initial calibration by the laboratory but can then be compared between different centres. In ECMO support, a multicentre consensus appears to be obtaining anti-Xa levels of 0.3–0.5 IU/mL. These are lower than the levels required when treating venous thrombosis (0.5–1.0 IU/mL).
Thromboelastography
Thromboelastography is a functional test that measures the viscoelastic properties of blood and evaluates the whole clotting system, including platelet function, clotting factors and fibrinolysis.
The use of thromboelastography during ECMO can sometimes be useful in bleeding patients, but it will not detect some antiplatelet effects (it will usually be normal in patients on aspirin). The addition of various reagents such as heparinase facilitates the interpretation of thromboelastography.
How important are all these tests?
Specialist input will save lives, but this is not always obvious to the bedside clinician.
This can be shown in the example of a patient admitted with acute heart failure following CPR and established on ECMO. A first dose of heparin was administered on cannulation and an aPTT obtained several hours later showed an APR of 4.8. The heparin dose was progressively decreased and the APR levelled at 1.9. Despite what was thought adequate anticoagulation, multiple thrombi developed in the cardiac chambers. Anti-Xa levels were found to be disproportionally low in relation to APR, and further testing revealed a deficiency in factor XI. Multimodal monitoring would have detected this anomaly earlier.
Standard decision trees should be developed to support staff at the bedside. This should include when to call the haematology department (see example in Figure 7.3).
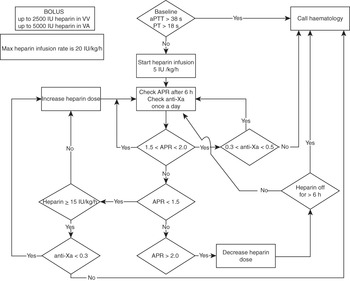
Figure 7.3 Example of a decision tree to support staff at the bedside. VA, veno-arterial; VV, veno-venous; PT, prothrombin time.
Blood product transfusion
Major blood losses can happen in patients supported by ECMO, and provision of compatible units of blood and blood products should be immediately available.
Some patients may receive repeated transfusions, and up to 4% of them will develop red blood cell antibodies. This means that compatibility tests should be repeated, usually every 72 h. The presence of antibodies requires repeated cross-matching of the patient’s serum to detect the emergence of additional antibodies.
Women of child-bearing age who are rhesus negative must be given special attention, and haematology should always be involved to decide whether it is justified to give anti-D serum.
Abnormalities of blood count
Thrombocytopenia
A large number of critically ill patients experience thrombocytopenia. A very severe numerical thrombocytopenia may show only minimal bleeding. Thrombocytopenia can be caused by reduced production or increased destruction of platelets.
Platelets are produced in response to thrombopoietin secreted by the liver. Platelets can vary considerably in size. Generally, large platelets are more active than small platelets, and are more efficient in thrombus formation.
The main treatment for thrombocytopenia is platelet transfusion. The majority of thrombocytopenic patients recover their platelet count when ECMO is discontinued.
Clinicians’ opinion on the minimum platelet threshold that is safe is very variable and ranges from a platelet count of 20 × 109 to 150 × 109. This difference is not confined to the field of ECMO.
Clinicians should measure the increment of a platelet transfusion. A satisfactory platelet increment is an increase by at least 10 × 109/L measured 60 min after infusion. Inconsistent increments between platelet transfusions may alert the clinician to HLA immunization.
Platelets have minimal ABO-system antigen expression but express HLA class I antigens. The HLA class I antigens can immunize the patients and cause refractoriness to certain donors but not others. It is possible to source HLA-matched platelets.
Immunization of HLA can occur in pregnancy or through red blood cell transfusion, and therefore can be observed in all patients, even if never exposed to platelet transfusion.
Heparin-induced thrombocytopenia
Heparin-induced thrombocytopenia (HIT) is a rare complication of using heparin and can be seen in ECMO patients. It is caused by antibodies that are also frequently found in patients exposed to unfractionated heparin during ECMO support. Only a minority of patients will have platelet-activating antibodies.
The diagnosis remains a clinical diagnosis. A laboratory test has a high negative-predictive value (so it is very unlikely to be HIT even if you thought it was) but a low positive-predictive value (so it is unlikely to be HIT if you thought it was not). Confirmatory methods require great expertise and are only available in selected centres, which causes major delays.
Most of the equipment in common use in ECMO is heparin coated. Heparin-free ECMO is a major challenge and may not be possible for the patient who has been diagnosed with HIT.
Outcomes vary, and generally the prognosis of HIT on ECMO is poor unless ECMO can be discontinued and heparin-free anticoagulation commenced. Heparin-coated material and in situ platelet transfusion should be kept to a minimum and only be considered in life-threatening situations after the systemic administration of heparin has been discontinued. Removal of the heparin-coated material is the only solution and this will allow the reaction to subside. Alternative anticoagulation can then be instituted, but haematology expert advice must be sought as there is a greater risk of coagulation imbalance leading to thrombosis.
Anticoagulation of a patient on ECMO with anticoagulant other than heparin is possible. The main challenge is the irreversible nature of the anticoagulant.
Other causes of thrombocytopenia
The cause of thrombocytopenias in the ECMO patient often cannot be identified, but some can be treated, so it is always worthwhile seeking specialist advice.
Acute or ‘acute on chronic’ immune thrombocytopenia justifies the administration of prednisolone and intravenous immunoglobulin. Immunoglobulins may sometimes be useful to contain thrombocytopenia observed after transfusion or transplant, which is often markedly more haemorrhagic than immune thrombocytopenia.
Drug-induced thrombocytopenia can be profound and should be excluded.
Thrombocytopenia can be related to the primary insult, justifying the use of ECMO. Thrombotic thrombocytopenia purpura is rare and is characterized by fever, intravascular microangiopathic haemolysis, thrombocytopenia, and renal and central nervous system symptoms caused by hyaline thrombi in the brain and kidney (but not in the lungs). Sepsis and haemophagocytic syndrome may present with extremes of pancytopenia.
A bone marrow biopsy may be helpful, particularly in patients presenting with thrombocytopenia. This can be performed safely on a thrombocytopenic patient, even if supported with ECMO.
Arbitrary transfusion thresholds are often unachievable in patients with multifactorial platelet consumption. Empirical approaches can be adopted, such as platelet transfusions every 12 h and intravenous administration of immunoglobulins or use of HLA class I-matched platelets, even in the presence of a negative antibody screen.
Other changes in the blood film
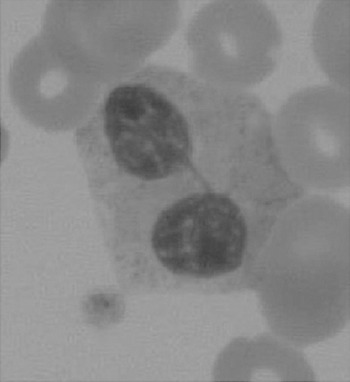
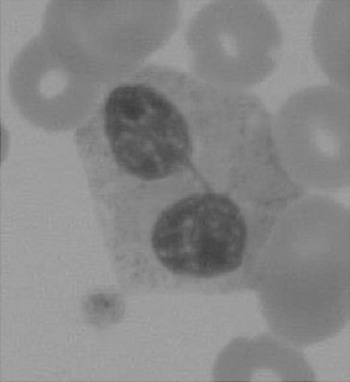
The haematologist will see multiple changes on the blood films. One such example is shown in Figure 7.4.
Neutrophilia, monocytosis and lymphopenia are common findings, especially during acute exacerbations of septic episodes. The white cells are often affected by the drugs being administered.
Haemolysis
Intravascular or extracorporeal haemolysis is a frequent complication of ECMO.
Haemolysis can be monitored by examining the blood film and measuring lactic dehydrogenase, haptoglobin and plasma-free haemoglobin. High levels of free haemoglobin can interfere with the optical instruments used in the laboratory to measure enzyme levels or coagulation tests.
Significant haemolysis may lead to anaemia followed by inappropriate investigations if not readily recognized.
Severe intravascular haemolysis should be investigated, and causes other than ECMO should be suspected.
ECMO-related haemolysis can be decreased by appropriate circuit management and aiming for the lowest possible blood flow. Multiple thrombosis in the circuit can lead to haemolysis, and replacement of the oxygenator can be a simple solution. Folic acid supplementation may alleviate symptoms.
Haemoglobinopathy patients
Special care has to be taken in the care of haemoglobinopathy patients. They should only be transfused with Rh- and Kell-matched blood products, and the full genotype match for Rh should be obtained to decrease the risk of antibody formation.
Sickle cell patients should, in addition, only be transfused sickle cell haemoglobin (HbS)-negative blood. About 10% of sickle cell patients may require cover with intravenous immunoglobulins and/or steroids if previously declared not transfusable. Frequent exchange transfusion to maintain the HbS level at less than 30% while critically ill is possible in sickle cell patients supported on ECMO.
Bleeding in patients on ECMO
Bleeding is an important cause of morbidity and mortality in patients on ECMO.
Minor bleeding (e.g. at the site of the cannula, line insertions or chest drains) is common in patients on ECMO. Minor bleeding often does not require any intervention other than local pressure. Persistent ‘minor’ bleeding (e.g. from a neck line suture) may result in significant volume loss if left unchecked for many hours and should not be left untreated.
External visible bleeding (e.g. from the site of a chest drain) should prompt assessment for occult bleeding (e.g. into the pleural space).
Major bleeding is a less common but serious complication. Gastrointestinal bleeding occurs in 5% and pulmonary haemorrhage in 10% of adults receiving ECMO support for respiratory failure.
Intracranial haemorrhage occurs in less than 5% of patients but is a feared complication of ECMO. Neurosurgical consultation is advised and, while most cases are not amenable to surgical correction, it should not be considered a reason to end support with ECMO. Veno-venous ECMO can be continued for weeks without any other anticoagulant than the heparin coating on the circuit, and some patients fully recover.
Measures to prevent bleeding include close attention to anticoagulation and judicious administration of blood products. Avoidance of any unnecessary surgical procedures is the most important way to prevent bleeding.
Any invasive procedures, whether in the operating theatre (e.g. thoracotomy) or at the bedside (e.g. vascular access, chest drain insertion, tracheostomy), including seemingly ‘minor’ procedures (e.g. nasogastric tube insertion, transoesophageal echocardiography), can all be the cause of significant bleeding.
Lumbar puncture is contraindicated. Intramuscular injections and venepuncture for blood sampling should be avoided
Management of bleeding in patients on ECMO involves optimization of coagulation using blood products, administration of drugs and surgical correction of the cause of bleeding. A suggested outline is given in Table 7.1.
Table 7.1 Management of bleeding in patients on ECMO involving optimization of coagulation using blood products, administration of drugs and surgical correction of the cause of bleeding
The first step is to rapidly assess the degree of haemorrhage. Major bleeding with haemodynamic compromise should prompt urgent intravascular volume resuscitation, ideally with cross-matched blood, and may require early surgical intervention.
Continued bleeding, despite optimization of platelet count, coagulation parameters and corrective surgery where indicated, should prompt further haematological input.
There is little trial evidence for the use of specific drug therapy in the management of bleeding in patients on ECMO, and support for particular agents is based on anecdotal reports.
Lysine analogues such as tranexamic acid and aminocaproic acid can prove useful in cases of fibrinolysis. Fibrinolysis that occurs suddenly in a patient on ECMO can sometimes be corrected by changing the oxygenator. The most likely reason is the build-up of small thrombi in the oxygenator fibres leading to continuous activation of fibrinolysis.
Administration of recombinant factor VIIa is possible but may be associated with higher morbidity, as seen in patients undergoing cardiothoracic surgery.
Surgical intervention may be necessary to remove large haematomas acting as a continued trigger for fibrinolysis. Surgery should be carried out by an appropriately experienced practitioner, with meticulous attention to haemostasis. In difficult-to-control bleeding, packing followed by a scheduled return to theatre may be necessary, allowing time for correction of clotting abnormalities, hypothermia and acidosis.
Key points
Heparin is the main anticoagulant used in ECMO patients.
The optimal dose of heparin is unknown.
A combination of haematological tests should be used in ECMO patients, and the haematologist consulted on many occasions.