The scope of perioperative medicine
Perioperative medicine describes the practice of patient-centred, multidisciplinary, and integrated medical care of patients from the moment of contemplation of surgery until full recovery (Reference Grocott and MythenGrocott & Mythen, 2015). This encompasses the three stages of surgical care: preoperative, intraoperative, and postoperative.
This definition covers a wide range of patients with many different conditions, ranging from a low-risk, young, healthy person undergoing minor surgery in an ambulatory care setting to a high-risk older person with multiple co-morbidities undergoing major and complex surgery.
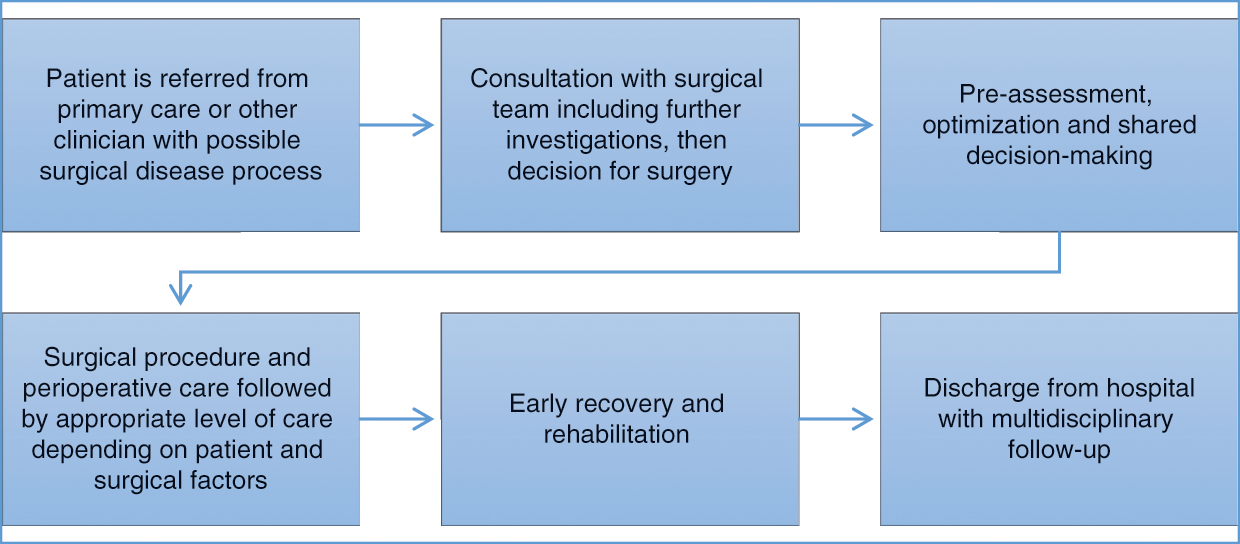
Perioperative care also involves a range of settings and disciplines. For the purpose of this chapter, it is taken as encompassing the period after a person with a possible surgical condition is referred to hospital by a primary care provider or ambulatory specialist, through traditional perioperative care, most commonly undertaken within a hospital, to their discharge and full recovery, as shown in Figure 8.1.

Figure 8.1 Patient pathway for elective surgery
Historically, the care provided to the surgical patient has been focused on the type of procedure being undertaken and the immediate recovery period, under the responsibility of an individual practitioner, a surgeon. It has typically been viewed in isolation from other elements of the patient’s experience, with little coordination and communication either within or beyond the hospital setting. However, reflecting a number of emerging factors that will be explored in this chapter, this model of care is being transformed to one that is individualized, coordinated, and delivers high quality care centred on the needs of the patient. This is particularly the case for high-risk patients with complex medical and social needs, undergoing major elective or emergency surgery. A major driver of the evolving model of perioperative care is the fact that patients with significant co-morbidities are increasingly being referred for surgical treatment. In the past these people would have been considered too high risk, or would have had a shorter life expectancy as a result of their medical conditions.
Over the last 10 to 20 years there has been a paradigm shift in the way that surgical patients are managed, driven by a mix of wider societal and clinical factors. During this time demand for surgery has risen considerably. According to OECD data, for example, in Denmark the rate of hip replacements was 140 per 100 000 population in 1996 but rose to 215 per 100 000 population in 2010 (OECD, 2015). A similar increase was observed in other western European countries, such as the Netherlands, but the increase was more pronounced in some of the southern European countries such as Greece, where it rose from 33.6 per 100 000 population in 1996 to 152 per 100 000 population in 2010.
During this time productivity has increased, driven in part by the increase in day-case surgery. The average length of stay (ALOS) (all causes) decreased across many European countries between 2000 and 2010. For example, the decline in the Netherlands was from 8.5 to 5.6 days, in the United Kingdom from 9.5 to 7.4 days, and in Greece from 8 to 6.6 days.
There is, however, considerable inter-country variation in length of stay. For example, the ALOS (for all types of patient) in Sweden was 15% lower than in the United Kingdom in 2011, with France having an ALOS 20% lower and Norway 36% lower. There are many reasons for this, reflecting different health system structures, organizations and economic contexts; however, the variation suggests that there may be opportunities to reduce length of stay in some settings, thereby potentially increasing productivity and making better use of available capacity. For example, widespread uptake of enhanced recovery programmes which combine a range of techniques to facilitate early discharge, and improvements in surgical techniques and care pathways which allow ambulatory surgery to be performed, both have the potential to dramatically impact productivity and efficiency. The impact of these changes could be significant; if ALOS in England fell by 15% by 2023, for example, with no further reductions in beds and all other things being equal, the NHS could treat around 18% more acute patients than it did in 2013/14 – an average annual increase of around 1.6% (Reference AlderwickAlderwick et al., 2015). However, as the cost of a patient recovering in a hospital bed is much less than that of undertaking a surgical procedure, the total cost would increase, possibly substantially.
Currently about 10 million patients undergo a surgical procedure in the English NHS each year, with consistent rises year on year, with a 27% increase seen in the number of surgical admissions between 2003/4 and 2013/14 (Royal College of Surgeons of England, 2017). The cost of elective (non-emergency) surgical care to the system is £16 billion (€20 billion). Out of these, around 250 000 patients are characterized as high risk (see below for a discussion of risk), representing 15% of all those who require inpatient surgical care and 80% of post-operative deaths (Royal College of Anaesthetists, 2016). Much of this risk is due to pre-existing long-term conditions and complex care needs, with the number of people living with multiple long-term conditions increasing steeply with age (Reference BarnettBarnett et al., 2012) and thus growing with an ageing population. For example, the 1.25 million people in the United Kingdom aged 85 or older are expected to treble in number over the next 35 years, and across Europe to rise from 5.1% of the population in 2014 to 12.2% by 2070 (Reference WilkinsonWilkinson et al., 2012; Eurostat, 2017). In England the number of people with multiple long-term conditions was expected to reach 2.9 million out of a population of 53 million by 2018 (5.5%) (Department of Health, 2012).
Improvements in perioperative management and surgical techniques have increased the numbers of people with pre-existing conditions deemed eligible for surgery and have, paradoxically, also been driven by the need to reduce the risk of complications. However, in many countries there is evidence of implicit ageism, with older people less likely to receive surgical interventions (Reference MarguliesMargulies et al., 1993). For example, a 2014 report showed that in England there was a 37-fold difference in rates of breast excision in patients with breast cancer over the age of 65, depending on where they live (Royal College of Surgeons & Age UK, 2014). This has led to calls to focus on physiological rather than chronological age (Reference KowdleyKowdley et al., 2012). However, among those who do receive surgery, older physiological age may be associated with a greater risk of complications which, when superimposed upon their already compromised physical state, mean that they may experience significant reductions in survival in the medium and longer term, and in their ability to return to their pre-operative function. Consequently, it is increasingly important that the scope of perioperative care extends beyond the immediate period of recovery from the acute effects of surgery.
This calls for a model of care that extends across specialties and professional groups and over time. Although the concept of perioperative medicine has been in use for more than a decade, until recently it has been applied only in a few selected areas and, even then, often incompletely. One area where it has been used is in cardiac surgery, where many facilities have established mechanisms to deliver efficient, multidisciplinary, patient-centred care. In contrast, most surgical specialties lack a unified approach to the prevention and management of perioperative surgical, medical, psychological, and social complications.
This is changing, with new models of perioperative care that emphasize improvement and consistency of outcomes for patients after surgery (Reference Kehlet, Delaney and HillKehlet, Delaney & Hill, 2015). These are fundamentally multidisciplinary, led by professionals who can take a system-wide approach and who can be drawn from a range of medical specialties, but most often anaesthesia, surgery, geriatric or internal medicine. This chapter will explore these models in detail and suggest opportunities and barriers to their future development.
The role of perioperative care
Perioperative medicine aims to deliver the best, multidisciplinary, person-centred care before, during and after surgery. There is a natural tendency to focus on major surgical interventions for the highest risk patients; however, the evolving models of care can be of benefit to the entire surgical population. As the vignettes in Box 8.1 reveal, there is huge variation in the perioperative care provided.
Box 8.1 Patient stories: traditional versus integrated care
Patient story: traditional non-integrated care
Stan is 72 years old with a history of high blood pressure and diabetes. He is a heavy smoker. He goes to his primary care provider as he has been losing weight recently and suffering with stomach pains. His GP refers him urgently to a surgeon, who does some further tests and confirms that Stan has bowel cancer. He recommends that he undergoes surgery and a few days later Stan comes back to the hospital to the pre-assessment clinic (PAC) and sees an anaesthetist, who is concerned that he may have chronic airways disease and that his diabetes is poorly controlled. The anaesthetist refers the patient back to the GP for further investigations but due to the urgent need for surgery, Stan arrives on the day of the operation without record of these tests. The surgery goes well and the cancer is removed; however, two days later Stan develops a chest infection and spends three days in the high dependency unit. His recovery is further complicated by a wound infection. After six weeks Stan is discharged from hospital to a rehabilitation facility and then home, where he requires carers three times per day.
Patient story: integrated care
Ruby is 81 years old with a history of cardiac disease and chronic kidney impairment. Following her complaints of symptoms of abdominal pain and bloating, her GP orders some blood tests and scans which raise the suspicion of ovarian cancer. She is referred to a “one-stop shop” clinic which takes place in a local health centre; there, she has a consultation with a surgeon and cancer specialist who offer her surgery and chemotherapy. On the same day she has further tests to assess her fitness for surgery, followed by a consultation with a cardiologist and an anaesthetist, where a shared decision is made to proceed to surgery. Records are kept electronically and shared with Ruby’s care providers. She is supported by a specialist cancer nurse who provides her with a single point of contact and coordinates her care. The operation goes well and she is electively cared for in the high dependency unit in order to provide early detection and treatment of any complications. Ruby’s recovery is uneventful and she returns home 10 days after the surgery, to begin chemotherapy shortly afterwards.
Perioperative care is often poorly coordinated, with weak systems of communication, focused on the individual practitioner and existing organizational structures. At worst, vital information about the patient is not shared between practitioners, resulting in untimely or delayed care and errors. Also, patients may undergo procedures in circumstances where they have not been made fully aware of the implications, resulting in dissatisfaction, poor outcomes, and a worsening of their general health status. However, where good perioperative medicine exists, the care provided is focused on the needs of the patient, employing individualized care pathways. The care is well coordinated and timely, and patients share in the decision-making process. Models of care vary (as explored below) but have common themes:
Multidisciplinary: involving doctors (both primary and secondary care), nurses, allied health care professionals, such as physiotherapists, occupational therapists, speech and language therapists and dieticians, social workers and administrative staff.
Crossing organizational interfaces: particularly primary care, secondary care and social care (Reference JohnsonJohnson et al., 2013).
Well led: this could be by doctors from different specialties, including anaesthesia, surgery, acute medicine, cardiology, geriatrics and others. Most commonly, anaesthetists lead perioperative teams since they are the most numerous hospital specialty and their current training model makes them natural candidates to do so. However, the interdisciplinary nature of good care means that there should be an emphasis on deploying the skills and expertise available in order to achieve optimal patient outcomes.
Robust communication: through the provision of a single point of contact for patients, surgeons and primary care providers, facilitated where possible by technology that enables the secure collection and exchange of patient data.
Evidenced-based with continual improvements in quality driven by robust audit data.
Patient-centred: respecting patients’ autonomy, listening to and respecting their wishes, and keeping them informed and involved with their care are the key tenets of patient-centred care which is now widely seen as an essential component of gold standard practice (Reference Epstein and StreetEpstein & Street, 2011). This model of care is gradually superseding its antiquated predecessors – doctor- and disease-centred care – and represents a paradigm shift from the patriarchal style of medicine which was practised for much of the 20th century.
Using appropriate technology: at present the majority of perioperative care is delivered in a visit-based system with the patients travelling to a hospital/clinic to be reviewed by the health professional who provided the index treatment. With the rise of digital health platforms and the ever-increasing availability of technology, there is potential for increasing amounts of perioperative care to be delivered remotely in a home-based system.
Multidisciplinary assessment and optimization – models of care
Geriatrician-led
Pre-operative CGA provided by a consultant geriatrician-led MDT involves multidomain assessment and optimization of the condition of the high-risk or older surgical patient (Reference PartridgePartridge et al., 2014; Reference MougMoug et al., 2016). This is particularly important given the increased frequency of risk factors and adverse post-operative outcomes in the older patient. The MDT can also support the surgical teams with post-operative medical care, focusing on functional optimization and discharge planning for both emergency and elective patients.
There is growing evidence that CGA is associated with improved process and outcomes such as decreased length of stay, reduction in delays and cancellations, and reduction in medical complications. One example is the Proactive care of Older People undergoing Surgery team (POPS) at Guy’s and St Thomas’ NHS Foundation Trust in London (Reference DhesiDhesi, 2012). The POPS team was designed to improve perioperative care and planning, address problems with poor rehabilitation and delayed discharges, and reduce the high rates of post-operative medical complications in elderly patients.
The Medical Research Council framework for complex interventions (Reference CraigCraig et al., 2008) was used to create, implement, and evaluate the POPS team. It is a geriatrician-led MDT which includes anaesthetic and surgical teams, therapists, social workers, and nursing staff. Patients with multiple co-morbidities, frailty and/or cognitive impairment are identified and referred to the POPS team. A CGA is then performed and a personalized perioperative care plan generated. Pre-operatively, risk factors and co-morbid conditions are identified and optimized, discussions are held with the patient, their family and the MDT to aid shared decision-making, and the appropriate level of post-operative care is determined. Post-operatively, regular geriatrician reviews and ward rounds take place and cases are discussed at POPS MDT meetings; there is also close communication between the POPS team and community/social services, facilitating quick and effective discharge to the community.
Around 1000 elective patients are seen by the POPS team annually, and the team also reviews any appropriate patients admitted to the surgical wards as an emergency. The impact of this service has been impressive, with significant reductions in medical complications, including pneumonia and delirium, pressure sores and delayed mobilization, and in length of stay in hospital (Reference Dhesi and SwartDhesi & Swart, 2016). Similar findings were obtained with the Systematic Care Older Patients undergoing Elective Surgery (SCOPES) service at Nottingham University Hospitals NHS Trust in England (Reference Dhesi and SwartDhesi & Swart, 2016).
Anaesthetist-led
Patients are triaged (based on estimated perioperative risk of mortality), with higher-risk patients attending an anaesthetist-led clinic. The clinician employs a range of clinical assessment and physiological testing (e.g. cardiopulmonary exercise testing) to provide an objective assessment of the risks and benefits of surgery. The clinic is supported by a range of health care professionals to provide expert advice and support (including organ specialists, therapists, and allied health care professionals).
Assessment of fitness for surgery
The assessment of fitness for surgery, and therefore risk of post-operative complications, is fundamental to perioperative care. There is strong evidence for an association of objectively measured fitness with outcomes from major surgery: in general, fitter people do better and this is perhaps even more important than chronological age (Reference SnowdenSnowden et al., 2013).
Assessing fitness allows an assessment of risk, thereby facilitating a discussion leading to a shared decision about whether and how the patient should proceed to treatment. Simple methods have been used to gauge cardiorespiratory fitness, for example using patient questionnaires to ascertain the person’s maximal level of daily activity and the 6-Minute Walk Test where the distance walked by the person predicts morbidity and mortality.
Reliably and objectively testing and quantifying fitness is increasingly becoming a prerequisite for major elective surgery, particularly in those patients known to have risk factors such as chronic diseases or obesity. Cardiopulmonary exercise testing (CPET) uses an incremental exercise test (usually on a treadmill or exercise bike) to generate safe, accurate, and repeatable data that correspond with the demands of major surgery on the body (Reference Carlisle J and SwartCarlisle & Swart, 2007).
Barriers to greater use of CPET include the costs of setting it up, the routine operation of the equipment, and the need for skilled expertise to conduct the assessments and interpret the test results. Often the test is conducted by physiologists, supported by clinicians. Many anaesthetists are now trained to make these assessments and there is growing recognition that the cost of managing post-operative deterioration in patients who have not been thoroughly assessed and their condition optimized often outweighs the costs of providing the tests.
Assessment of fitness can be done as part of comprehensive pre-operative screening. This can be nurse-led and most hospitals in England also have consultant anaesthetist-led clinics to assess more complex patients. At this point in the patient journey blood investigations and assessments of the function of other body systems (heart, lungs and kidneys) are also done and patients may be referred for specialist opinions.
Historically, pre-operative testing was largely performed on the day before surgery and it was left to the admitting junior doctor to decide which tests should be performed, leading to significant variability in pre-operative testing and creating the potential for significant patient harm. With the shift towards PACs this process has become more rigorous and standardized, with significant improvements to patient care. PAC is now widely accepted as the gold standard of care across Europe, exemplified by a law passed in 1994 in France which stipulates that a PAC visit must be completed at least two days before any admission for elective anaesthesia (Reference Flynn and SilvayFlynn & Silvay, 2012). However, the PAC approach has its own pitfalls as there is a tendency towards over-testing and delays to treatment as incidental abnormalities are followed up and investigated further. The recognition of risk of patient harm due to unnecessary investigations, and delayed definitive treatment of the initial pathology, have led to a trend of more selective pre-operative testing (Reference FeelyFeely et al., 2013; Reference Bohmer, Wappler and ZwisslerBohmer, Wappler & Zwissler, 2014).
This is exemplified by the joint recommendations from the German societies of Anaesthesiology, Internal Medicine, and Surgery (DGAI, DGIM, and DGCH), published in 2010. These recommendations highlight the importance of precise medical history and examination, and suggest a standardized scheme to identify factors which may necessitate further testing. If there are no such factors and the procedure to be performed is low risk, the authors claim that no further testing is needed. The recommendations address patient- and procedure-specific indications for pre-operative testing such as laboratory tests, electrocardiogram, X-ray, echocardiogram, pulmonary function and extended cardiac testing. The aim is to reduce unnecessary investigations which have been shown to have no beneficial effect on perioperative patient safety, thereby streamlining the pre-operative assessment process and reducing costs and delays to treatment. A national survey of German anaesthesiologists performed in 2013 suggests that the recommendations have been effective, with 39.1% of anaesthetists stating that they now conduct fewer ancillary tests (Reference Dhesi and SwartDhesi & Swart, 2016).
Risk optimization and lifestyle modification
Once a comprehensive assessment of risk has been undertaken, and as part of the multidisciplinary approach, measures to optimize the chances of a good outcome from surgery can be decided in collaboration with the patient. Through liaison with other professionals, control of chronic diseases such as diabetes, asthma and heart disease can be optimized. In addition, lifestyle advice can be given and other services can be sign-posted, including smoking cessation (Reference McKee, Gilmore and NovotnyMcKee, Gilmore & Novotny, 2003), alcohol reduction, weight loss, and dietary and nutrition advice.
Recently, the concept of “prehabilitation” has been adopted; this consists of a group of interventions that are introduced into the patient pathway pre-operatively, aimed at enhancing a person’s ability to withstand the stress of major surgery and achieving lasting beneficial effects on recovery (Reference GillisGillis et al., 2014). Although the choice of timing must be balanced with the risk of delaying surgery (particularly in cases where cancer is suspected or diagnosed), it is evident that improvement in pre-operative fitness will optimize the chances of a successful outcome from surgery.
One major intervention, with increasing evidence of benefit (although not consistently), is exercise therapy (Reference Snowden and MintoSnowden & Minto, 2015). There is overwhelming evidence that physical activity improves the health of people with chronic conditions and also prevents many common diseases (Academy of Medical Royal Colleges, 2015). This is also true in the context of the pre-operative phase but it is important that an exercise programme achieves a high level of adherence, with support from the appropriate health professionals. Several studies have shown significant improvements in length of stay and reductions in post-operative complications following cardiac surgery in patients who have used prehabilitation programmes (Reference HoogeboomHoogeboom et al., 2014). There is also some evidence that prehabilitation can benefit patients undergoing thoracic, abdominal and major joint surgery, particularly in high-risk patients with poor pre-operative condition (Reference HoogeboomHoogeboom et al., 2014).
Unfortunately, the current body of evidence surrounding prehabilitation is skewed towards low-powered randomized controlled trials in healthy individuals, whereas the greatest benefit is likely to be seen in high-risk patients. Furthermore, there is a lack of consensus regarding the most efficacious exercise programme, for example whether it should be resistance or aerobic training, and whether it should be delivered in a hospital or home-based environment (Reference HoogeboomHoogeboom et al., 2014).
Pre-operative risk assessment and shared decision-making
The concept of shared decision-making (SDM) is attracting increasing attention in many countries (Reference BlancBlanc et al., 2014). It represents a shift from antiquated paternalistic medicine to a patient-centred model, and is especially pertinent in the field of perioperative medicine as decisions surrounding surgeries can have life-changing consequences.
SDM is defined as “a broad term that describes [a] collaborative effort between the physician and patient to make an informed clinical decision that enhances the chance of treatment success as defined by each individual patient’s preferences and values” (Reference Slover, Shue and KoenigSlover, Shue & Koenig, 2012). It involves the provider offering information on possible treatment modalities, including risks, benefits and alternatives, and the patient sharing their relevant values and preferences. A mutual decision can then be made on a treatment plan most likely to deliver the best outcome with respect to these factors, whether it is a choice between different types of surgery, or a choice between surgery and conservative management. This type of patient empowerment has several benefits, including decreased indecision and decisional conflict, and improved patient knowledge and participation in treatment decisions. It allows for care to be tailored to the needs of individual patients and can increase patient satisfaction.
Box 8.2 Patient story: shared decision-making
Anil is 78 years old and undergoes routine screening for abdominal aortic aneurysm (AAA). This reveals that he has an 8 cm aneurysm and so is referred to a vascular surgeon. Since the risk of it rupturing is around 50% per year, Anil is offered an open surgical repair and is then seen in a PAC. Anil also has heart failure and emphysema, and his health has been deteriorating for a while. In the clinic he undergoes a CPET, among other tests, which reveals that he has a poor physiological reserve. Following this, he has an hour-long discussion with an anaesthetist, where the risks and benefits of having surgery are discussed. Anil understands that he is at high risk of complications if he has surgery, and will be unlikely to get back to his pre-operative level of function. Following a period of time to reflect and discuss with his family, he returns to the clinic and decides, along with his care providers, not to proceed with surgery and instead to adopt a conservative approach.
SDM has been shown to affect patient decision-making, with a tendency to choose more conservative therapeutic options, particularly in orthopaedic patients (Reference Slover, Shue and KoenigSlover, Shue & Koenig, 2012). It has also been postulated that it can improve equity in health care, as the physician is beholden to explain alternative treatments that may have been unknown to certain groups of patients (Reference ElwynElwyn et al., 2010).
Although SDM is increasing and is seen as the gold standard of patient care, uptake has been limited, due in part to the perception that it is an expensive and time-consuming endeavour that requires an investment in training. However, the impact on consultation time is usually minimal, and the growth of digital technology means that decision-making aids can be produced and disseminated at relatively low cost (Reference ElwynElwyn et al., 2010).
Box 8.3 Torbay Hospital Clinic shows financial viability
The surgical risk assessment and SDM clinic at Torbay Hospital, South Devon Healthcare NHS Foundation Trust, UK, is an excellent example of SDM in operation. Approximately 900 high-risk patients per annum are referred to the SDM clinic to have a comprehensive risk assessment and an in-depth consultation regarding their treatment options. The aim is to empower patients to make more informed decisions on their care and to allow perioperative care planning including allocation of resources such as high dependency and intensive therapy units. This model has been shown to be financially viable, with an estimated £382 (€480) reduction in total cost of care for high-risk patients undergoing bowel cancer resection.
Care bundles – enhanced recovery
In recent years enhanced recovery programmes (ERP) have become increasingly popular, with a substantial body of evidence demonstrating their ability to improve post-operative outcomes and to reduce length of stay. Common components of ERP include pre-operative counselling, planning, and nutrition, usually delivered in an outpatient clinic setting, and after the patient has been admitted to hospital intra-operative management such as guided fluid therapy, maintenance of normothermia (a normal state of temperature) and use of minimally invasive approaches. Post-operatively, initiatives such as early mobilization, prompt resumption of normal diet, innovative analgesic techniques and proactive discharge planning are employed.
Enhanced recovery after surgery (ERAS) for colorectal surgery was first described by Professor Henrik Kehlet in Denmark during the 1990s (Reference FearonFearon et al., 2005). The principles of this programme are shown in Figure 8.2. Subsequently the same elements have been applied to other surgical specialties, including orthopaedics and gynaecology, and have developed into the international ERAS society with centres of excellence in Canada, Denmark, France, Spain, Sweden and the United Kingdom.

Figure 8.2 Components of enhanced recovery after surgery (ERAS) pathway
Note: NSAIDs: non-steroidal anti-inflammatory drugs
There have been several studies showing improved outcomes, such as length of stay and morbidity, when ERAS is used (Reference AdaminaAdamina et al., 2011). Despite this body of evidence, uptake of the programme and adherence to its principles have been relatively low; patient-, staff- and practice-related factors as well as a lack of resources have been suggested as potential barriers to entry which must be overcome if widespread implementation is to be achieved (Reference Segelman and NygrenSegelman & Nygren, 2014). Furthermore, there is a paucity of evidence on the effect of ERAS on patient-related outcomes such as quality of life and cost-effectiveness, and furtherresearch is indicated in these areas to quantify the true value of ERAS and other ERPs.
Research on post-operative outcomes in orthopaedic patients after use of ERPs has been promising; however, there is a wide variation in the components of the programmes evaluated, with substantial variations in results (Reference IbrahimIbrahim et al., 2013). Development and widespread implementation of a standardized enhanced recovery protocol would help in disseminating best practice. Reference StowersStowers et al. (2014) suggested a protocol for enhanced recovery after hip and knee arthroplasty described in Table 8.1 below, which shares many features and principles of the ERAS protocol for colorectal surgery while being tailored towards the needs of patients undergoing orthopaedic surgery.
Table 8.1 A proposed enhanced recovery protocol for elective total hip and knee arthroplasty
| Pre-operative care |
|
| Intra-operative care |
|
| Post-operative care |
|
Ambulatory surgery
Over the past 20 years there has been a significant increase in productivity driven by the rise in proportion of operations performed as ambulatory cases. Since 2005 England has employed financial incentives to switch to ambulatory surgery, driven by the rollout of a system of payment by results (PbR) for all elective procedures. As day-case patients cost less to treat than patients who stay overnight as inpatients (in 2013/14 the average day-case cost was £698 (€872) and the average inpatient-case cost was £1367 (€1708)), the increasing number and proportion of day cases has helped to reduce overall costs per case. In effect, by treating more patients as day cases, by 2013/14 the NHS had saved around £2 billion (€2.5 billion), equivalent to an average saving over the 15 years since 1998/9 of around 1.4% per year of the total spend on elective day and inpatient care (Reference ApplebyAppleby, 2015).
Several other factors have facilitated the shift towards day surgery including: cultural change, availability of regional anaesthesia, faster-acting anaesthetic, analgesic (pain-killer) and antiemetic (anti-sickness) drugs, organizational improvements, i.e. day-case units, minimally invasive surgery, and changing patient expectations.
Workforce
Current anaesthetic workforce model
In most countries anaesthetists form the largest single hospital medical specialty and their skills are used in all aspects of patient care (Royal College of Anaesthetists, 2016). While the perioperative anaesthetic care of the surgical patient is the core of specialty work, the scope of anaesthetic practice can extend to:
The pre-operative preparation of surgical patients
The resuscitation and stabilization of patients in the ED;
Pain relief in labour and obstetric anaesthesia;
Intensive care medicine, although increasingly this is becoming a specialty in its own right with a separate training and accreditation structure;
Varying age groups: neonatal, paediatric and adult;
Transport of acutely ill and injured patients;
Pain medicine;
The provision of sedation and anaesthesia for patients undergoing various procedures outside the operating theatre.
In the main, services are delivered by specialists, placing large demands on the current workforce in some countries. With an ageing population, many with multiple co-morbidities and requiring more complex surgical procedures, there are projections of at least a 25% increase in demand in the United Kingdom by 2033 (Centre for Workforce Intelligence, 2015).
Non-physician anaesthetists
In some European countries anaesthesia is currently delivered by non-physicians, albeit with supervision by consultants (Reference VickersVickers, 2000). Box 8.4 illustrates some examples.
Box 8.4 Employment of non-physicians to give anaesthetics
Sweden: Anaesthetic nurses (ANs) are all drawn from nursing backgrounds. They may enter AN training directly after graduating as a nurse, although most also have a minimum of two years’ practical nursing experience. The AN training programme lasts for one year. Physicians supervise a variable number of theatres and for the most part physicians must be present at the induction and reversal of anaesthesia.
The Netherlands: Anaesthetic nurses are drawn from either nursing backgrounds or straight from school with good exam results; the former group undergo two years’ training and the latter three years’ training. Physicians normally supervise two operating theatres and must be present at the induction and reversal of anaesthesia. An AN must be present at every anaesthetic.
The United Kingdom: The main groups eligible to commence training as a physician’s assistant (anaesthesia) or PA(A) are registered health care professionals with at least three years’ clinical experience and/or degree level studies, or graduates with a biomedical science or biological science degree. Typically PA(A)s work in a 2:1 model where there is one consultant anaesthetist supervising two PA(A)s or a trainee anaesthetist and a PA(A) simultaneously in two operating theatres. PA(A)s are also used to reduce theatre downtime, leading to increased throughput on lists and theatre utilization, pre-operative assessment, exercise testing, provision of sedation to other specialties, cardiac arrest teams, and for regional and local anaesthetic provision. This model has not, however, been widely adopted, with only around 120 PA(A)s trained by 2015, but this is projected to increase with plans by the Department for Health and Social Care to fully regulate PA(A)s.
Perioperative care workforce model
As has been outlined above, optimal perioperative care is delivered by a well led MDT, focused around the patient. In many acute care settings the components of the team already exist but are often fragmented and exist in isolation with poor communication between them. Members of the perioperative MDT include:
Doctors (both primary and secondary care), including:
➢ Anaesthetists
➢ Surgeons
➢ General practitioners
➢ Care of the Elderly physicians
➢ Specialist physicians such as diabetologists, cardiologists and respiratory physicians
➢ Radiologists
➢ Intensivists
Nursing staff
Physicians’ assistants
Allied health care professionals such as:
➢ Physiotherapists
➢ Occupational therapists
➢ Speech and language therapists
➢ Dieticians
➢ Social workers
Administrative staff
Training
High quality and well organized training is integral to the future of perioperative care. Clinical training for physician anaesthetists combines the acquisition of clinical knowledge, skills and behaviours, with a broad range of clinical leadership and management skills necessary. In addition, clinicians are now increasingly required to have at least a working knowledge of improvement science, discussed in more detail later in the chapter, and the ability to apply relevant research into their clinical practice.
As has been discussed, good quality perioperative care transcends traditional boundaries in terms of clinical specialties and across organizational forms. This requires that training adapts too, whereby clinicians from different specialties such as anaesthesia, surgery and medicine acquire similar skills and knowledge in order to collaborate more closely. Post-graduate qualifications, such as the UCL Perioperative Medicine MSc, are open to all health care professionals thus promoting true multidisciplinary working (https://www.ucl.ac.uk/surgery/courses/msc-perioperative-medicine).
Barriers to delivery of perioperative care
There is a projected shortfall of physician anaesthetists, as well as other specialties. In the United Kingdom changes to medical and nursing training has resulted in a deficit of applications for training posts, meaning that some roles within the perioperative team are left unfilled. This threatens the sustainability of the workforce and poses safety challenges in terms of rota gaps, unmet service need, and increased requirement for locum or ad-hoc positions. Recently, there has also been difficulty filling positions for higher training in anaesthesia (http://www.rcoa .ac.uk/news-and-bulletin/rcoa-news-and-statements/rcoa-links-low-fill-rates-inadequate-supply-of-trainees). However, this situation may offer an opportunity for the design and implementation of new models of care (as discussed below) and improved patient outcomes.
Good quality perioperative care transcends traditional organizational forms and systems. For example, patients will often be cared for by their primary surgical team in conjunction with other medical and non-medical specialists in primary and secondary care. This demands good communication. Due to difficulties sharing information in health systems, however, information is often not passed on or made available when it is required. This can lead to replication, waste and, at worst, error. In addition, further barriers to the provision of good quality care, as with other health care settings, include inter- and intra-provider variation, processes lacking reliability, and lack of standardization. Addressing these issues is best done using improvement science principles (see below).
The future
Health care systems are facing challenges from the ageing population with a greater prevalence of chronic co-morbid conditions, and the opportunities to intervene provided by advances in medicine. However, with these challenges comes the opportunity to innovate and implement transformational change to the way that we deliver perioperative care. Consideration also needs to be given to the appropriateness of costly, complex surgical therapies, and whether centrally funded health care systems should be expected to provide these with the possible consequence of less available resources for more established therapies with proven cost-effectiveness. Policy-makers have a responsibility to engage with the public in discussion, and as a society, in order to determine where each health care system’s priorities lie within a cost-constrained environment.
For the vast majority of patients undergoing a surgical procedure, the episode is uncomplicated with good outcomes. However, increasing numbers of patients are being exposed to greater risk through a combination of their pre-existing condition, the surgical treatment itself, or issues regarding the delivery of care. The development of the perioperative care model offers a solution that can optimize the chances of a good outcome, particularly for high-risk patients.
Excellent perioperative care is, in part, already being offered in an individualized manner with the ability to draw on expertise and resource, as and when the patient needs it. This is described in Figure 8.3.
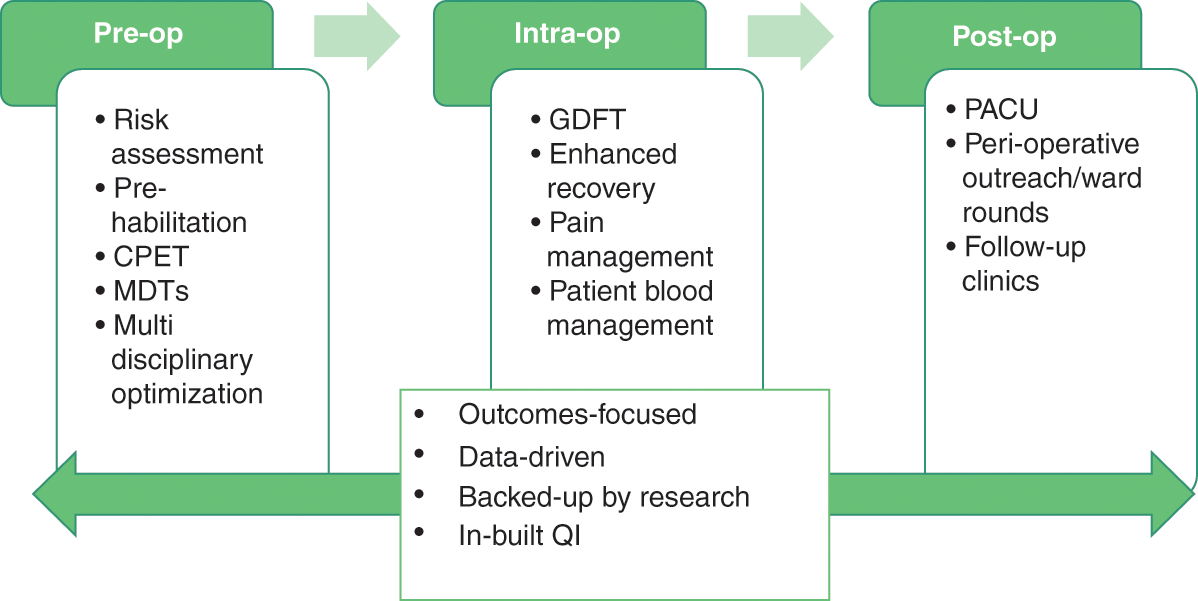
Figure 8.3 Individual perioperative care pathway
Notes: CPET: cardiopulmonary exercise testing; MDT: multidisciplinary team; GDFT: goal directed fluid therapy; PACU: post-anaesthesia care unit; QI: quality improvement
There are a number of enablers to the provision of quality, coordinated perioperative care including: technology, research and improvement science, and improved models of care.
Technology
Although the use of technology in medicine is growing, we have yet to truly tap into its full potential. Advances in genomics, telemedicine, robotics, virtual and augmented reality, artificial intelligence and electronic medical records have the potential to cause a paradigm shift in the delivery of perioperative care. As these advances in computing continue at an exponential rate, the challenge for perioperative care providers is to find new and effective ways to harness technology to improve outcomes for their patients. However, this is often costly and demands front-loaded funding. Even if this results in cost-savings and efficiencies in the medium to longer term, financial cuts mean that technology programmes face significant challenges.
Increasingly data are being digitized, which can then be analysed, shared and used to drive quality improvement. For example, powerful machine-learning algorithms could be applied to ascertain which patient, provider and procedural characteristics will impact most on their post-operative outcomes, or be used to supply live decision support to PACs enabling selection of appropriate pre-operative tests and a bespoke prehabilitation package. Digitized data can be seamlessly and securely transferred between stakeholders, including hospitals, primary care providers, research and academic institutions, and patients themselves. This increased availability of information presents manifold opportunities for research and identification of best practice, allows for safer and more efficient delivery of care through the avoidance of repetitive data gathering, and can empower patients by giving them ownership of their medical records.
Innovation in anaesthesia in the past 10 years has centred on a number of aspects:
Airway equipment, for example video laryngoscopy.
Ultrasound machines, which are now in widespread use in anaesthesia for use in diagnosis, vascular access and regional anaesthesia where needles are inserted under direct vision and local anaesthetic drugs are deposited around nerves.
The increasing profile given to human factors and systems design, particularly in the management of clinically challenging, time-sensitive situations. The Clinical Human Factors Group (CHFG), founded by Martin Bromiley, a pilot whose wife died as a direct result of medical error, is at the forefront of this (Clinical Human Factors Group, 2018); mitigation of these important sources of error and risk to patients has been increasingly recognized as having a significant impact in perioperative care. Techniques implemented to help control human factors include: application of learning from other sectors, such as the aviation industry, human factors design and engineering, and improved simulation and team working techniques (Reference Weinger and GabaWeinger & Gaba, 2014).
Drugs, especially those that enable enhanced recovery; for example, sugammadex is a novel reversal agent for some muscle relaxant drugs, although its use is limited by its relatively high cost.
Increasing awareness around the environmental impact of anaesthesia, particularly that of the volatile agents and nitrous oxide, which are greenhouse gases, is driving increased use of total intravenous anaesthesia (TIVA). There is also increasing evidence that TIVA with propofol is associated with decreased reoccurence of malignancy following cancer surgery, the mechanism of which is unclear.
Research and improvement science
The evolution of perioperative medicine needs to occur in parallel with the development of the research agenda, with a particular focus on translating discoveries and advances into meaningful changes in care delivery and outcomes for patients more rapidly. At present, basic scientists are directing their efforts at understanding the biological mechanisms underlying post-operative morbidity, and why its impact should be so sustained. Clinical triallists are evaluating interventions to mitigate adverse outcomes in pragmatic studies involving tens of thousands of patients. It is recognized that unplanned variations in structures and processes between health care providers have a significant impact on outcomes after surgery; thus initiatives within the field of improvement science are focusing on this area.
Improvement science in health care is a concept that has been generating increasing interest over the past few years, as health care providers, academics, and front-line staff look to improve care delivery and generate practical real-life learning and approaches to aid development and dissemination of best practice. However, it is still in what some authors call the “pre-paradigm phase of emergence”, which in part means there is an absence of an agreed definition (Reference Marshall, Pronovost and Dixon-WoodsMarshall, Pronovost & Dixon-Woods, 2013). Commonly the term is used to describe the application of the principles of W Edwards Deming to health care. A broader definition of improvement science is that it is a coordinated approach to quality improvement (QI), which aims to create practical learning that can make a timely difference to patient care (Reference Marshall, Pronovost and Dixon-WoodsMarshall, Pronovost & Dixon-Woods, 2013).
Improvement science is built around the robust scientific assessment of QI projects, including the design, deployment, and assessment of complex multifaceted interventions. If applied correctly, it adds considerable external validity to the results of these interventions, allowing them to be taken up more rapidly by other institutions and health care systems, and breaking down silos of best practice. The process of rapid testing and improvement helps to generate confidence in the proposed changes among the stakeholders.
Furthermore, this approach helps to mitigate the risks caused by poorly planned and unscientific QI projects, which are not evidence-based, nor appropriately monitored to ensure positive impact on patients. Therefore improvement science is critical to maximizing the impact of QI interventions and effective use of resources as health care systems adjust to the demands of modern and future medicine (Reference Varkey, Reller and ResarVarkey, Reller & Resar, 2007).
There has been a lot of research looking at QI interventions in perioperative care. This is because although significant advances have been made in recent years, there are an estimated 234 million surgical procedures performed annually around the world with considerable risk of patient harm. A recent systematic review of QI research in perioperative care using techniques such as audit and feedback, Plan-Do-Study-Act (PDSA) cycles, and methodologies such as Lean Six Sigma which are used to remove waste and reduce variation, demonstrated that although there were many studies in this field, the reporting was suboptimal, leading the authors to conclude that we need to orientate research towards QI and improvement science in perioperative care and develop a comprehensive, coherent, and valid framework for the design and reporting of QI interventions in this field (Reference JonesJones et al., 2014).
Recognition at all levels of health care from policy-makers, commissioners, and organizational boards to front-line staff that QI should be part of an organization’s daily business is essential in order that a culture of continuous improvement is sustained. Improvement work performed as part of teams is most effective but in order for this to occur, it is important that time and resources are dedicated to it; however, in many instances, in part due to the sustained pressures of delivering against rising demands, QI is regarded as a non-mandatory activity.
Fundamental to developing a supportive and nurturing culture that encourages innovation and improvement is the adoption of coaching. An example of an effective health care system that has embedded coaching into its systems is the Sheffield Microsystem Coaching Academy (Sheffield Microsystem Coaching Academy, 2018).
Collaboration between academics and clinicians is flourishing with the recognition that “big data” and nationally funded audits of processes and outcomes can be used to study and deliver improvements in these outcomes.
Developing evidence can be combined with significant advances in technology, digital health, patient empowerment and anaesthetic techniques to produce gold standard models of care. These models of care and existing examples of best practice should be scaled across health care systems in order to reduce variability in standards of care delivered and to improve patient outcomes.
Improved models of care
In the immediate future efforts to improve perioperative care should include the dissemination of existing best practice – for example, enhanced recovery programmes have been shown to improve post-operative outcomes; however, their use has remained sporadic. This is a prime example of where best practice, validated by research, could be scaled to positively impact the lives of vast numbers of patients. These programmes have the potential to bring greater improvements by taking a more holistic approach, including nutrition and prehabilitation, and by utilizing the power of technology to improve patient engagement.
Perioperative care could also be rapidly improved by the uptake and dissemination of shared decision-making principles, empowering patients to take more charge of their care journeys, and putting patient preference at the centre of perioperative care. Where digital patient information resources are created, these should, where possible, be made open source and widely disseminated to spread best practice in a cost-effective manner.
As we redesign our services and meet the demands of 21st-century medicine, it is important to embrace the truest form of disruption, which is taking techniques and learning from different sectors and applying it in innovative ways to solve the problems we face. One good example of this would be the application of engineering and manufacturing principles such as lean methodology to health care systems. This would develop superior, more efficient processes, with fewer delays for the patient and higher productivity for the hospital, and consequently free up capacity to treat more patients and generate more funding (Reference Dahlgaard, Pettersen and Dahlgaard-ParkDahlgaard, Pettersen & Dahlgaard-Park, 2011), which could then be reinvested in order to fund the array of technologies discussed elsewhere in this chapter. Furthermore, when we are implementing new models of care or improving existing ones, it is important that we utilize the improvement science techniques described above in order to ensure maximum efficiency and continuous improvement, and create data with external validity.
When health care providers look further ahead and plan delivery of perioperative care in the mid-21st century, it is important that they embrace the shift towards patient-centred, home-based care, and integrate the necessary infrastructure to utilize the myriad of technological advances that are already presenting themselves (Reference RosenRosen et al., 2016).
It is possible that much preoperative assessment could be completed remotely through the use of telemedicine consultations, at home diagnostic equipment, and digital educational resources to deliver prehabilitation and relevant information for the patient. This type of remote working will free up space in hospitals and will allow health care professionals to work more efficiently, but it also require substantial staff training, organizational culture change, and investment in the necessary equipment and software to make it a reality.
The operating theatres of the future should allow for advanced surgical equipment such as robotics and imaging devices. Digital connectivity will be paramount to allow incorporation of remote multidisciplinary input, access to electronic health records, and integration of machine learning and artificial intelligence clinical decision-making and technical assistance tools. Robotic surgery has also created the interesting concept of remote operating; conceivably the principal surgeon could operate from a console thousands of miles away from the patient, allowing their expertise to be shared on a global scale. Fully autonomous robotic operating devoid of any requirement for human input is viewed by many authors as being the future of surgery, with the potential to become the standard operative modality and revolutionize perioperative care (Reference MoustrisMoustris et al., 2011).
The transition to these improved models of care will be challenging and, due to the level of infrastructural improvements required, will be likely to require substantial up-front investment. However, there are some favourable societal trends emerging, for instance the general public are increasingly becoming digitally connected, with most households in developed countries now having internet access, and smartphones and other devices being readily available. This technological environment is perfectly primed to connect patients and health care providers and can facilitate the patient-centric and home-based care of the 21st century.
Furthermore, the previously discussed challenges that health care is currently facing, with rising demand for services and financial constraints, represent significant drivers for change; the need to innovate in order to improve efficiency and modernize care delivery has never been greater. This is well demonstrated in the United Kingdom by the NHS five-year forward view policy document (NHS England, 2016), which puts innovation and new models of care at centre stage.
The scope of imaging
Radiology is constantly evolving in its clinical application, playing a central role in numerous patient pathways in health care. Advances in sophisticated technologies have extended the scope of its application to every organ, offering not only essential services in diagnosis, monitoring treatment, and predicting outcomes but more recently therapy in the form of interventional radiology. The result of these developments is that the volume of activity is continuing to grow in all imaging techniques (often referred to as imaging modalities).
The term “imaging” encompasses a number of diagnostic tests, some of which may be performed outside a radiology department. There is great variation among countries and by specialty in how these processes are undertaken and where.
Imaging was originally founded on the plain X-ray. Despite the development of newer techniques towards the latter part of the 20th century, the plain X-ray still plays an important role in diagnosis (although its role is often to rule out pathology, rather than for primary diagnosis) and its uses continue to grow. However, the newer modalities of ultrasound, CT and MRI are increasing at a more rapid rate. Figure 9.1 shows the increased activity in England in the last 20 years. This demonstrates a 3.6% compound growth in the last five years.
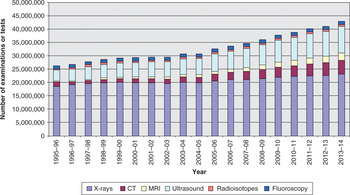
Figure 9.1 Total number of imaging and radiodiagnostic examinations or tests, by imaging modality, England, 1995–96 to 2013–14
Major growth can be observed in the more complex cross-sectional imaging techniques, with compound annual growth rates (CAGR) in the last 10 years of 10% for CT and 12.3% for MRI (see Table 9.1). There is slightly less growth recorded in ultrasound at 5.3%, but this may be an underestimate as a significant amount of ultrasound is now performed outside imaging departments and would therefore not be recorded in these figures.
Table 9.1 Compound annual growth rates (CAGR) for radiology modalities in England
| Modality | CAGR for past 5 years | CAGR for past 10 years |
|---|---|---|
| X-rays | 1.46% | 1.40% |
| CT | 9.13% | 10.05% |
| MRI | 9.70% | 12.32% |
| Ultrasound | 5.72% | 5.32% |
| Radio-isotopes | 0.25% | 0.70% |
| Fluoroscopy | 1.23% | 0.90% |
Although these figures are specific for England, a similar picture is seen throughout Europe and internationally. This growth is significantly in excess of that expected by demographic drivers and is predominantly due to the increased reliance on imaging particularly in areas such as cancer, vascular conditions (including stroke and cardiac disease), and trauma.
As well as the established diagnostic techniques, imaging continues to expand at pace particularly focusing on the concept of molecular imaging utilizing ultrastructural diagnostics, nanotechnology, and functional and quantitative diagnostics. The main example of this in current practice is the use of fusion imaging, which combines the structural information gained from CT (or MRI) with the functional information from positron-emitting radiopharmaceuticals in the form of positron emission tomography fused with computed tomography (better known as PET-CT). The result is the depiction of the spatial distribution of specific metabolic or biochemical activity with clear anatomical localization.
This improved image clarity and tissue differentiation in a number of situations has dramatically increased the range of diagnostic information, in many cases providing increased confidence in terms of underlying pathology. These fused images are vital tools in a number of clinical areas, notably cancer diagnosis and treatment, but they are also used in neuroimaging and cardiac imaging (Box 9.1).
Molecular imaging is rapidly gaining recognition as the future direction of imaging providing information of what is happening at the molecular/cellular level in terms of both structure and function. The main techniques currently in clinical practice utilize radiopharmaceuticals to provide functional information combined with traditional scanning techniques to provide structural information. However, there is active research into other techniques utilizing optical imaging for instance. The current research suggests that this form of imaging combined with genomics may be able to provide more personalized focused imaging in terms of earlier diagnosis, particularly in the field of cancer care, and allow more selective, effective treatment management.
One of the most significant changes in radiology in the last 20 years has not come from developments in imaging techniques. Rather, the technological advances in information technology (IT) have had a major impact on the way that radiology is currently practised. The days of viewing X-rays on sheets of film are in the past. These days, when images are acquired, they can be post-processed, manipulated and also transmitted rapidly not just within a hospital but also anywhere in the world as soon as they have been acquired. This technology, referred to as picture archiving and communication systems (PACS), has challenged the traditional model of patient, scanner and radiologist all located in the same site (Box 9.2). Images can now be reviewed and reported from remote locations, opening up options for different delivery models.
Box 9.2 Picture archiving and communication systems
PACS (picture archiving and communication systems) is a health care technology for the short- and long-term storage, retrieval, management, distribution and presentation of medical images. PACS allows a health care organization (such as a hospital) to capture, store, view, and share all types of images internally and externally.
A PACS has four major components:
imaging systems, such as MRI, CT or X-ray equipment
a secure network for distribution and exchange of patient information
workstations or mobile devices for viewing, processing, and interpreting images
archives for storage and retrieval of images and related documentation and reports.
PACS has been a major driver for changing the way imaging services are delivered. The electronic storage and transfer of images facilitates quick and easy access to images and reports. In addition it has allowed the radiologist to review the images at a site remote from their acquisition, giving rise to teleradiology as a new concept.
So far in this chapter, the emphasis has been on the diagnostic role of imaging. However, imaging can also be used to guide therapy, a specialty referred to as interventional radiology (IR) (Box 9.3). This is now established as an alternative to conventional surgery in numerous conditions, offering less invasive alternatives with improved outcomes, safety, and cost-effectiveness, as well as more patient-focused care. As such, IR is a vital component of hospital medicine, providing life-saving care, both in and out of hours. IR services have replaced or enhanced many surgical procedures as well as allowing new treatments for patients which were not previously feasible. Interventional radiologists are part of the multiprofessional teams treating a wide range of pathologies and working closely with surgical colleagues.
Box 9.3 Interventional radiology
The impact of interventional radiology
Aortic aneurysm: Rupture of the abdominal and thoracic aorta can be prevented and treated by the insertion of covered stents, which have largely replaced conventional surgery for this condition. In some cases these procedures are now carried out under local anaesthesia.
Gastrointestinal haemorrhage: Embolization therapy is increasingly performed by interventional radiologists for the control of uncontrolled bleeding from the lower and upper gastrointestinal tract. This life-saving procedure carries a much lower risk to the patient and in many cases is the treatment of choice.
Postpartum haemorrhage: Bleeding after childbirth remains the most common cause of maternal death and the role of IR in managing this emergency is well established.
Cancer: By using minimally invasive techniques, early cancers can be destroyed using radiofrequency or cryotherapy. Patients avoid the need for major surgery and long-term outcomes are very favourable. Newer techniques allow selective radiotherapy or chemotherapy for the treatment of liver lesions. Embolization can be used to devascularize tumours prior to surgical resection with resulting improvements in safety.
Early management of stroke: In the early stages of stroke the infusion of thrombolytic agents dissolves the clot and mechanical removal of blood clots can be performed to minimize disability and reduce the risk of death. Patients who suffer stroke from subarachnoid haemorrhage (bleeding around the brain) are now most frequently treated by interventional radiologists using embolization techniques.
Renal obstruction: Obstruction of the outflow from the kidney is frequently complicated by infection, which leads to septicaemia (infection in the bloodstream) and risk of death. Interventional radiologists are able to bypass the obstruction, for example through percutaneous nephrostomies.
The interconnections of imaging in the hospital setting
Imaging plays a significant role in most hospital-based specialties. The exact workload of an imaging department depends, to a certain extent, on the clinical specialties available within the hospital (e.g. neurosurgery, oncology).
In UK hospitals, A&E and general practice (direct access) are the specialties with the highest radiology demand, followed by Trauma and Orthopaedics, and this makes up approximately 50% of the activity. There is further demand from other specialities such as general surgery, general medicine, obstetrics and gynaecology, rheumatology, geriatrics, gastroenterology, cardiology, thoracic medicine, vascular surgery, ophthalmology, ENT, neurosurgery, neurology, paediatrics, oncology, psychiatry and intensive care.
The model of imaging provision varies throughout Europe. In many countries the hospital-based imaging department remains the main provider of imaging for emergency and urgent care, as well as planned care and community services. As discussed in the next section, in some countries the demand from primary care and from office-based practice is met by imaging services based off-site from acute hospitals.
In looking at new models of delivery, it may be more useful to consider where imaging plays a role in patient pathways and at what stage in this pathway imaging is best accessed. Table 9.2 is not exhaustive but lists the more common pathways and presentations relying on imaging.
Table 9.2 Common pathways and presentations relying on imaging
| Suspected or diagnosed cancer | Breast, brain and neuro-axis, head and neck, lung, oesophagus and stomach, colon and rectum, liver, pancreas, kidney and ureter, bladder, prostate, testes, ovary, uterus and cervix, lymphoma, musculoskeletal, melanoma |
| Cardiovascular disease | Chest pain, heart failure, pulmonary embolism, venous thromboembolism, aortic aneurysm, peripheral vascular disease |
| Respiratory disease | Chest infection/pneumonia, chronic obstructive pulmonary disease, restrictive lung disease |
| Head and neck | Deafness, balance disorders, tinnitus, sinus disease, thyroid disease, visual disturbances incl. field defects |
| Neurological conditions | Acute stroke, transient ischaemic attack, headache, epilepsy, multiple sclerosis, dementia, Parkinson’s disease and other movement disorders |
| Trauma | Head injury, fractures, chest and abdominal injury |
| Musculoskeletal | Back pain, myelopathy and radiculopathy, joint pain, osteoarthritis, rheumatoid arthritis |
| Pregnancy | |
| Genito-urinary | Renal failure, renal stone disease, renal tract obstruction, pelvic mass, pelvic pain, haematuria |
| Endocrinology | Hypertension, Cushing’s disease, adrenal disease |
| Surgical | Acute “surgical” abdomen, paediatric surgical conditions |
Diagnostic radiology does not just offer an image acquisition and reporting service. Radiologists work closely with their clinical colleagues to ensure that patients get the most appropriate investigation and that the interpretation of the report is understood in relation to the clinical context. In this role, the radiologist plays an important part in the MDT approach to patient care, which has been acknowledged as a significant factor in improving outcomes, particularly in cancer care (Reference MorrisMorris et al., 2006; Reference StephensStephens et al., 2006; Reference CooryCoory et al., 2008). This has led to the development of MDT meetings where clinical radiologists (who usually lead the meetings) with their diagnostic pathologist colleagues work alongside their clinical colleagues to decide the correct clinical plan for each patient. These diagnostic specialists aid surgeons and oncologists in developing appropriate care plans based on the staging of the cancer. In this function it is now common for the biopsy of the primary tumour to have been performed by a radiologist under imaging guidance aided by the pathologist’s interpretation. Figure 9.2 illustrates the extent of these MDT meetings in a typical large hospital.
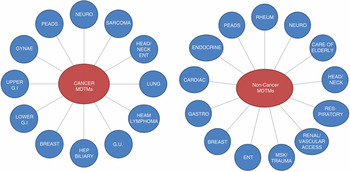
Figure 9.2 Multidisciplinary team meeting (MDTM) participants
IR also interacts with a large range of clinical services, as illustrated in Figure 9.3. The patients treated by interventional radiologists may be inpatients on wards in the hospital, but more frequently are treated as day cases. Larger imaging departments may have their own day-case facilities, but if not, the IR service needs access to such a resource.
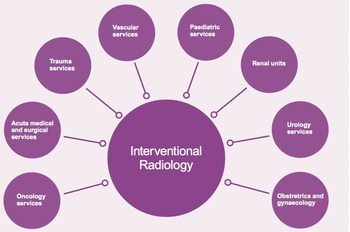
Figure 9.3 Interventional radiology interactions with hospital departments
Links with services outside hospitals
Patients access imaging services from a number of different situations, including:
hospital inpatients
outpatient services based in hospitals
consulting rooms outside hospitals
primary care doctors/health care professionals
self-referral.
Imaging activity referrals from outside the hospital setting are increasing significantly. This is influenced by a number of factors including a drive to earlier diagnosis of conditions such as cancer and heart disease (Independent Cancer Task Force, 2015), as well as the increasing capability to support patients to manage their health care outside the hospital. In areas such as plain X-ray and ultrasound the workload from primary care can often amount to over 50% of the imaging activity.
Although imaging is usually thought of as a tool to confirm a diagnosis, it is important to emphasize the role of the negative test in excluding significant disease. In many pathways early access to imaging can avoid unnecessary hospital outpatient appointments and, more importantly, unnecessary hospital admissions.
In many European countries there is direct access to imaging from primary care for all main modalities (i.e. CT, MRI, ultrasound and plain X-ray). This applies particularly to the field of musculoskeletal problems where there is high demand for MRI in the management of back pain and joint pain.
There are varying delivery models across Europe to meet these demands. In some countries the hospital imaging service also provides imaging services for referrals from outside the hospital, while in other countries much of this activity is provided in centres located outside hospitals either linked to or independent of the hospital departments. These centres may also provide services for “outpatient” imaging from specialists who work in office practice, notably in insurance- or private-based health care systems.
Workforce
There are two main clinical professions that deliver imaging in Europe: radiologists and radiographers.
A radiologist is a doctor who is also an imaging expert with specialized training in obtaining and interpreting medical images. As mentioned already, radiologists can also treat diseases by minimally invasive, image-guided surgery (interventional radiology). Like other doctors, a radiologist must first qualify as a doctor from an accredited medical school and spend a variable period in clinical practice. Following this, they will undertake further postgraduate training before qualifying as a radiologist (usually for a further five years in most European countries).
A radiographer (or medical imaging technologist) is a trained health professional whose primary role is to produce medical images that assist radiologists and other doctors to diagnose or monitor a patient’s injury or illness. In most European countries they have undergone training at degree level or equivalent followed by in-post further subspecialization. Some radiographers extend their role beyond that of image acquisition. This practice is more common in the United Kingdom than in most other European countries. Such activities include interpretation of ultrasound tests, mammography screening, and trauma plain film reporting.
The United Kingdom is probably the most advanced European country in developing a career progression in its radiology workforce through the development of four tiers of radiographer training and professional development. These include:
Assistant practitioner (not a trained radiographer): an assistant practitioner performs protocol-limited clinical tasks under the direction and supervision of a registered practitioner (radiographer).
Practitioner (state registered, degree educated): a practitioner autonomously performs a wide-ranging and complex clinical role, and is accountable for his or her own actions and for the actions of those they direct.
Advanced practitioner (state registered): an advanced practitioner, autonomous in clinical practice, defines the scope of practice of others and continuously develops clinical practice within a defined field.
Consultant practitioner (state registered): a consultant practitioner provides clinical leadership within a specialism, bringing strategic direction.
A smaller workforce of nurses, health care assistants, and physicists as well as administrative and clerical roles supports these two professional groups. The development of PACS is creating a key role for IT support.
The legislative and regulatory framework varies, particularly with ultrasound. For example, in many countries (including the United Kingdom), radiologists have little involvement in performing and interpreting obstetric ultrasound. The obstetric ultrasonographers may be radiographers who have trained specifically in this practice, but may also be obstetricians and midwives.
A similar picture can be seen, to varying degrees, in other specialties where clinicians have acquired their own ultrasound equipment and provide a focused ultrasound service to support their specialty interest, e.g. urology, orthopaedics, or vascular surgery. This practice is most advanced in cardiology, where the cardiologists have developed their own expertise to acquire and interpret images as well as carry out interventional procedures under radiological guidance. In the United Kingdom, for instance, the term echocardiography refers to ultrasound of the heart and is usually performed within the cardiology department by separately trained technicians under the supervision of cardiologists, while cardiologists, rather than radiologists, often report CT and MRI of the heart and great vessels. This may be carried out on separate dedicated scanners in large centres, but more commonly the radiographers in the main imaging department acquire the images.
Existing barriers to delivering optimal imaging services
As the role of imaging has gained greater importance in health care, there is a real challenge to respond to the increased demand due to a number of factors, which has led to significant variation in the use of radiology in Member States across Europe. Figure 9.4 illustrates the variation in CT and MRI activity across Europe.
The following challenges and barriers are thought to be the major influences on the current usage and effectiveness of imaging in Europe.
Evidence-based access to imaging
It is difficult to draw conclusions from a comparison of imaging activity between different countries, as there is a lack of evidence to indicate what the appropriate level should be and this will anyway vary with patterns of disease. In France and the United Kingdom, for example, national societies have developed evidence-based guidelines to encourage referring doctors to use imaging appropriately. These guidelines have been adopted by a number of other European countries with varying effectiveness (Reference RemediosRemedios et al., 2014; Royal College of Radiologists, 2016). The use of imaging tests involving radiation (CT, plain X-ray and nuclear medicine) is governed by European legislation in the form of the newly updated European Directive 2013/59/Euratom. This states, among other things, that all requests for such tests are “justified” by a responsible trained health care professional. The goal is to protect patients from unnecessary exposure to radiation. Despite this, there is evidence of an inappropriate over-usage of radiology in certain clinical situations. There is also likely to be overuse of MRI and ultrasound, although as these do not involve exposure to ionizing radiation, they are not governed by this regulation.
However, the concern does not just relate to possible over-usage of imaging. There is evidence suggesting the variation in cancer outcomes in Europe is partly due to variation in early access to imaging for diagnosis in suspected cancer.
Workforce issues
The marked growth in imaging activity in the last 10 years has been met with differing degrees of workforce expansion across Europe, but in most countries the increase in radiologists and to a lesser extent radiographers has lagged behind the growth in activity. The situation is most acute in those countries that started from a low base of radiologist per head of population. Figure 9.5 illustrates the variation in a number of European countries.
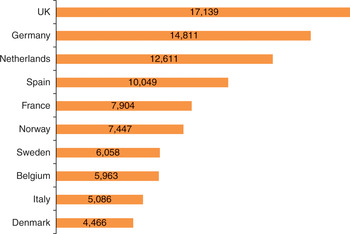
Figure 9.5 Number of inhabitants per radiologist, 2011 (including residents in training)
The situation with radiographers is not as acute, although in the United Kingdom, for instance, radiographers and ultrasonographers are included with radiologists on the government shortage occupational list for immigration purposes. This situation is mitigated somewhat by the United Kingdom approach to skill-mix, described earlier.
This shortage of radiologists is further compounded by the fact that they have developed additional roles, for instance in interventional radiology, the performance of biopsy techniques, and in their role in the MDT mentioned earlier. A recent survey from the European Society of Radiology found that 58% (out of 31 respondents) reported having too few radiologists for their current needs and 55% (out of 30 respondents) replied that they would not have enough radiologists in training to serve their respective nations.
In addition, there has been a drive to subspecialization with the increasing complexity of imaging techniques. This is creating a real challenge, particularly in smaller hospitals/services where it is proving increasingly difficult to provide an expert specialized opinion over seven days a week, throughout the year. Networking between hospitals is seen as a partial solution to this.
Equipment
There is also considerable variation in the equipment base across Europe. This is illustrated in Figure 9.6, which compares the provision of CT and MRI scanners in a number of European countries and international comparators.

Figure 9.6 Scanner equipment per million people in selected OECD countries, 2015 (or nearest year)
The international economic crisis has coincided with the recent rapid increase of imaging activity described earlier. This has significantly constrained the previous regular turnover of imaging equipment, resulting in a higher than usual amount of aged equipment, at a time when technological developments continue. This is leading to increasing levels of obsolescence.
This slow-down in equipment replacement is not only the direct result of shrinking budgets, but also the consequence of adaptive strategies leading to better use of resources. Radiology, based on highly technical hardware and diagnostic pathways, offers a fertile ground for workflow standardization resulting in productivity gains. Efficiency plans focus on a variety of measures including: merging of departments/hospitals, sharing of equipment, closing down excess capacity, policies for equipment upgrade, patient throughput optimization, and extension of opening hours. New equipment often offers improved imaging quality and reduced radiation exposure, due to the improvement of X-ray technology or to the substitution of non-ionizing technologies (e.g. MRI).
Funding issues
Within Europe, there are many different types of funding models for health care, mostly based on revenues from taxation or social insurance. There is also great diversity in how imaging is reimbursed. However, in the current economic climate, many facilities have faced substantial budgetary pressures, forcing reorganization of facilities, staffing arrangements, and equipment.
The reduction in budgets for radiology departments can give rise to a biphasic effect on volumes within a fee-for-service model. There can be an initial tendency to increase the volumes to compensate for the reduction in revenues resulting from the reduced procedural reimbursements, followed by a trend to reduce the volume of procedures requested, due to the attention paid to appropriateness.
In systems where there are global budgets, there is a tendency to increased utilization of low-cost techniques, such as ultrasound and plain radiography, to attempt to substitute for the higher costs of higher tech procedures, such as MRI and CT. This is one of the explanations for the disproportionate place of ultrasound and radiography in some countries, in comparison with more sophisticated imaging techniques.
Organizational constraints
As mentioned previously, there are varying models of imaging provision, which are influenced by a number of national and local drivers. The traditional model is that of imaging departments as part of an acute hospital and from this central base services are provided to the hospital and local community in terms of access to primary care requests. This has the advantage of centralization of high-cost equipment and skilled staff. However, the disadvantages include poor access for non-hospital patients and dealing with the competing agendas of acute/emergency care and planned/community care through one department. In many countries alternatives to this model have developed, ranging from provision of ultrasound services in primary care facilities, through mobile ultrasound, X-ray, CT and MRI services, to fully comprehensive planned care diagnostic centres (non-emergency) offering a full range of imaging services often alongside other health care activities (e.g. laboratory testing, consultation rooms, day-case procedures).
Hospital design and imaging service location
A key constraint to providing appropriate, responsive imaging services within a hospital is the location and design of the imaging department. Historically, the imaging department has usually been in one location with the possible exception of A&E, where there was provision for plain X-ray examinations.
As the role of imaging has developed as an integral part of the examination of the patient, the need to co-locate imaging equipment with certain clinical services has become essential. This can be solved relatively easily in the case of ultrasound but, in the absence of a new building, the relocation of MRI and CT scanners provides a considerable challenge. This is not just due to the problem of finding an appropriate space but is compounded by the specific radiation protection considerations for CT and equivalent safety considerations for MRI with its high magnetic field.
CT scanning should now be an integral service within an emergency department to deal with conditions such as acute trauma and stroke. In addition, as most inpatient scanning occurs within the first few hours of admission, it makes sense from both a patient-centred and efficiency approach to locate ultrasound, CT and MRI close to the admissions unit.
The problem is that many hospitals do not have a centralized admissions function and therefore the challenge of delivering this approach is often too great. This results in considerable movement of patients around the hospital, which at best results in a poor experience for the patients and at worst can delay management and in some situations raise safety issues.
There are similar drivers of patient-centredness and efficiency within the outpatient setting with an impetus to offer one-stop clinics, which include consultation, investigations, and sometimes treatment in one visit. Thus in certain specialties that are high users of diagnostics (e.g. orthopaedics, gastroenterology, gynaecology), it makes sense to ensure that imaging facilities are either in the clinic area or adjacent to it.
This need for co-location creates the challenge of ensuring an appropriate level of staffing for such equipment. To a certain extent the development of PACS has solved this problem for radiologist support, but there can still be considerable challenges for radiographer and technologist staffing with potential for redundancy of scanning time.
The future of imaging services
There is no evidence that the current increase in demand for imaging services is likely to reduce in the next five years. Recent work commissioned by Cancer Research UK suggests that the current increase in demand for CT, MRI and ultrasound will continue at the current rates (Reference Cancer ResearchCancer Research UK, 2015). Although there is less reliance on plain X-rays in certain areas of medicine, trauma and orthopaedics will continue to rely on skeletal plain films while the chest X-ray is unlikely to reduce in usage.
Research into the effectiveness of screening in ovarian and lung cancer will shortly be published. It is likely that this will suggest the introduction of screening programmes for at-risk patients. In lung cancer this would result in a further marked increase in CT of the chest.
One of the limiting factors to the expansion of the use of CT has been the risk of repeated radiation exposure, but the new generation of scanners has markedly reduced radiation levels. This is one of the drivers behind calls to evaluate lung cancer screening programmes and is likely to increase the use of CT in other presentations.
The usage of PET-CT will further increase in the next five years, predominantly in the area of cancer management, but the role of fusion imaging is likely to expand beyond cancer. One such area of expansion appears to be in neurological conditions, particularly dementia. Other forms of molecular imaging are most likely to remain as research tools in the next five years with no immediate plans for widespread use in clinical practice.
The scope of interventional radiology continues to expand. A proportion of IR has focused in the area of vascular disease. This will continue to expand with further applications in areas such as thrombectomy in the treatment of acute stroke. There is also likely to be a further major expansion in the use of interventional image-guided therapy in cancer care. This will extend beyond its established use in symptomatic relief and palliation. There are already a number of indications for its primary use in treatment (e.g. neo-adjuvant embolization, image-guided ablation and brachytherapy, trans-arterial chemo-embolization (TACE), selective internal radiation therapy (SIRT), and isolated perfusion chemotherapy).
The implication of current and future developments in imaging for the organization, management and design of the hospital in the mid-21st century
Although some of the functions of hospitals may change in the future, the management of emergency and urgent care will remain their primary focus. Imaging will continue to play a vital role in supporting this activity, with an increasing reliance on CT particularly for trauma and the acutely ill patient.
It is therefore important that the planning of imaging facilities is part of any planning of new emergency and admission departments in order to deliver timely, safe and efficient services. Imaging will also be required in high dependency areas such as intensive therapy units, high dependency units and certain wards. Thus, great thought needs to be put into hospital design to avoid inappropriate siting or unnecessary duplication of imaging facilities with resultant unnecessary redundancies.
It is likely, therefore, that imaging will no longer be housed in one department and consideration will need to be made in terms of staffing, particularly in facilities that need to be accessible 24/7. With the challenges of workforce supply, described earlier, it is essential that the efficient flow of patients through imaging is a key consideration in hospital design.
The challenge that imaging departments face in balancing the demands of emergency and urgent care with planned care could be addressed, but this is dependent on two main factors. The first of these relates to the future role of the acute hospital in dealing with planned care, particularly in the form of outpatient facilities. If these remain on the main hospital site, then there will need to be imaging provision alongside them, particularly where the concept of the one-stop visit is to be achieved.
The second factor is the demand for access to imaging from primary care. In this situation there is no need for the patient to attend the hospital and in fact there are definite advantages both to the patient and to the hospital if such visits can be avoided. This could be achieved by further development of imaging services outside hospitals. To make these cost-effective, they may need to be centralized in diagnostic centres for modalities such as CT and MRI, while ultrasound could potentially be delivered in GP surgeries if there is appropriate demand.
If such diagnostic centres were to be developed, they would also have the potential to provide an alternative facility for hospital outpatients, particularly if the diagnostic centres had facilities for consultations and minor procedures.
All hospitals that deal with emergency and acutely ill patients need access to interventional radiology. As with radiology, this must be available 24/7. Although the emergency work will require inpatient beds with full clinical support, there will also be a demand for planned procedures which can be performed on a day-case basis. Consequently, a facility that can deliver this should be located adjacent to the interventional suite. Although all hospital emergency Should this be ‘hospital emergency departments’ or ‘hospital emergency patients’? and inpatients will need access to interventional radiology services, it is unlikely that all acute hospitals of the future will have enough demand for such work to justify their own comprehensive funded service. The solution to this will be the development of various forms of network where either the patient is transferred to the experts (or possibly the experts travel between hospitals).
For radiology to continue to play a key role in health care it must be able to respond to the workforce needs and therefore adequate provision must be made for education and training.
Likewise for imaging to continue to develop and support health care in the future, adequate provision and funding of research involving imaging is essential and this is likely to be concentrated in larger hospitals. The provision of imaging is essential to much of medical research, particularly in the monitoring of new therapies. However, there is a need to carry out primary imaging research if the true potential of new technologies is to be achieved.
At present the radiology department remains predominantly the domain of the radiologist, but this is changing and there is no specific reason why other clinical specialists trained in imaging should not use imaging facilities (if possessing appropriate skills). If the case for this is established, then a coordinated imaging resource is far preferable to the growth of isolated services often with unused capacity and challenges of equality of access.
The opportunities and barriers to making this vision of the future a reality
As imaging continues to develop, it will remain heavily dependent on appropriate levels of workforce and equipment, but IT solutions have the potential to improve the efficiency of services. There are already electronic requesting systems in existence that are linked to evidence-based resources to aid the clinician in requesting the most appropriate test first time and avoiding unnecessary investigations and delays.
There is considerable interest and research into the use of artificial intelligence (AI) in the interpretation of imaging investigations. Although this is not yet at the stage of routine practice, it is likely that this will prove significant and may eventually substitute for radiologists in certain investigations.
As radiology increases in its complexity, it will be even more challenging for every hospital imaging department to employ enough radiologists to provide a comprehensive service throughout the week. One partial solution to this is the provision of efficient comprehensive PACS systems. This opens up options for transferring images in real time to radiologists outside the acquiring hospital. If used appropriately, this can facilitate the development of networks of expertise, which will support smaller hospitals and enable them to provide appropriate comprehensive services in a timely fashion. This can be particularly effective in the emergency situation, avoiding onerous rotas for small numbers of radiologists. An example where PACS can be used to provide support to such hospitals is that commonly seen in neuroradiology where a hub and spoke model often exists, with neuroradiologists supporting local radiologists with second opinions. There are also examples of network solutions where a group of subspecialized radiologists provide a service across a number of hospitals. In the emergency situation services now exist that offer radiologist reporting “out of hours”, removing the need for onerous on-call rotas for the local radiologists.
These solutions will not overcome the need for a significant increase in the radiologist workforce, but will help to ensure the effective use of radiologists.
There is an obvious need for this subspecialization radiological expertise within the hospital setting to provide expertise to the various clinical specialties that imaging supports. However, a great deal of radiology provision to primary care is of a relatively general nature and it will be important that adequate expertise remains to deal with this relatively large workload in a timely manner. Thus it will be important that radiology departments ensure that the development of subspecialization does not leave this important element of their work under-provided for. This is an area where the extended role of the radiographer may be a solution in some areas, particularly in the interpretation of the plain X-ray and in the performing and reporting of general ultrasound by sonographers. Some of their current work may be replaced by non-radiographer technologists working under their supervision.
Another challenge already mentioned is appropriate levels of imaging equipment. As there will be an increase in competition for space in existing hospitals, PACS also offers the solution to consider locating imaging equipment off the main site. This equipment, if sited effectively, could give better access to patients who do not require hospital facilities (e.g. outpatients and primary care patients). By decanting this work off the main site, it could have the added benefit of improving efficiencies in delivering inpatient imaging support.
Another issue to be considered, particularly in cross-sectional imaging (CT and MRI), is the increase in obesity in the European population. This has added another challenge to manufacturers who now have to consider increasing both the size of the machines and their weight limitations to accommodate the increasing number of patients who would not physically fit in the traditional scanners.
Finally, whatever innovative solutions are explored, these will not be effective unless an appropriate funding system is in place. This is obviously challenging in the current economic climate; however, there is no doubt that inadequate or inappropriate funding mechanisms have the potential to significantly hold back the effective use of imaging in the hospital of the future. It has to be realized that in many circumstances effective imaging services will deliver higher quality health care with efficiency savings elsewhere in the system, for example in reduced length of stay, avoidance of hospital admission, and reduction of unnecessary outpatient appointments.
Thus, it is essential that imaging services are an integral part of the planning of the hospital of the future to ensure that resources are used effectively and the potential improvements in both quality and efficiency of patient care are realized.
Introduction
Advances in laboratory medicine are happening at an uneven rate. On the one hand there has been a rapid expansion in innovative rapid molecular diagnostic techniques, but on the other hand translation into clinical impact has often been slow. Pathology services in many parts of Europe are undergoing modernization and reform but in some places this can be slow and patchy.
In this chapter, the terms pathology and laboratory medicine are used as synonyms to indicate cellular pathology, microbiology, virology, chemical pathology, immunology and haematology, molecular pathology, genetics and histocompatibility, and other laboratory-based medical specialties. As important as it is diverse, pathology is poised to become a key medical specialty, central to the development of stratified and personalized medicine, but it needs to overcome several challenges, not least the huge increase in complexity of tests, demand for digital data, the expectation of ever-reducing test costs, and shortages of trained staff. These issues as they impact upon European pathology are outlined with some specific national case studies.
The points below illustrate some of the emerging trends:
An unprecedented velocity of technological advance. Pathology services will continue to lead the transformation of medical care through, for example, genomics, proteomics, tandem mass spectrometry, and microarrays. These advances will have significant impact not only in the delivery of diagnostic and therapeutic services, but also in the workflow and ethos of patient care. Advances in technology, however, come at increased costs to organizations and health care consumers.
Self-testing and near-patient testing will proliferate. In parallel with advances in large-scale technology within laboratories there will be a proliferation in self-testing and single use devices to perform pathology tests outside the laboratory. The accuracy and reliability of these devices need to be vigorously examined, and capturing and storing the data generated by these devices might be problematic. There is a need to coordinate results from self-testing and point-of-care devices with the results from formal laboratories. The increased use of “wearable IT” with a health care purpose will raise expectations for seamless transmission of information to and from patients, and primary and secondary care providers, including pathologists. However, these devices, part of the “Internet of things”, raise concerns about data security, including both unauthorized access and commercial exploitation by software providers. Resolution of the confidentiality, privacy and security concerns will be led by patient or consumer demand.
Increasing collaboration and partnership is key. Greater interdisciplinary contact within medical specialties and subspecialties and between organizations is an inevitable consequence of the requirement to ensure high quality. For example, an integrated diagnostic service between pathologists and radiologists could speed up diagnoses, increase accuracy, and improve patient outcomes. Ensuring that pathology services are adjacent to clinical teams will be important to minimize risks to patients, but there is a need to understand better the options for remote working arising from videoconferencing and telepathology so that the right balance is achieved between clinical adjacencies, reducing unnecessary specimen transport, and achieving economies of scale.
Digital pathology is a disruptive technology. This development has great potential to make pathologists’ working lives more efficient, facilitate intra- and inter-departmental consultations, improve the efficiency and documentation of research, and enhance education and training. However, the adoption of digital pathology requires resolution of some longstanding issues. The time taken to scan slides, the significant storage required for the images, the capital cost of slide scanners and the variable costs associated with storage space, and sufficient data security will all need to be addressed as a priority.
The laboratory is a translational environment. For example, as clinical genomics moves from research to a routine diagnostic, prognostic and predictive method, this presents numerous challenges in terms of sample processing, quality control, and service developments in management and reporting. The knowledge base of pathologists trained and experienced in traditional methods will be tested by the need to provide and interpret the new reports. It is difficult for established pathologists, mostly based in traditional laboratories that do not provide the new tests, to add interpretation of these new tests to their repertoire. This may well require a new approach to learning which can integrate knowledge of clinical genomics into everyday practice. The implementation of new technologies tends to follow the Gartner Hype Cycle (Figure 10.1).
What do these advances mean for the role of the hospital in the future?
Laboratory medicine is the bridge between analysis and interpretation of clinical data and care delivery. Pathologists order, conduct, and interpret the results of hundreds of individual tests to support clinical decisions that enable good patient care. The laboratory’s role as a centre of diagnostics within the hospital of the future, however, may need to be redefined in the light of pressures for cost efficiencies, greater effectiveness and improved performance, and the impact of emerging technologies.
A common theme in European pathology is the quest for greater efficiency. In this respect, laboratories have looked towards increased automation to improve productivity and meet increased demand. Automation of the laboratory can lead to better task integration and quicker turnaround times. The argument follows that patients benefit as quicker clinical decisions can be made with the potential to shorten hospital stays.
Across Europe the modern laboratory environment is increasingly being organized to create networks based on large consolidated centres (hubs) and smaller local testing centres (spokes). A key driver for service reconfiguration has been cost pressures. In some countries laboratories face a bleak ultimatum: restructure or lose all your work. The key question, as Lord Carter put it in his Review of NHS Pathology Services in England (Reference CarterCarter et al., 2008), “What is the right level of consolidation?”
Another driver for change is the desire to reduce variation of diagnostic tests across countries both in test investigation costs and the over/under-requesting of tests. The United Kingdom Atlas of Variation, for example, shows that cancer patients who received an early-stage diagnosis – a critical factor in treatment outcome – ranged from 22.7% to 60.8% between the United Kingdom’s best- and worst-performing areas (Public Health Reference EnglandEngland, 2015).
Major advances in diagnostic laboratory medicine may be disruptive, as technical advances have the potential to provide a more efficient and cost-effective pathology service. For example, molecular diagnostic technologies are being utilized in a wide range of medical specialties including genetics, infectious disease, oncology and haematology. Their advantages have been well documented and allow for the simultaneous sequencing of many millions of individual DNA molecules. Using this technology, pathologists and researchers are provided with increased sensitivity and specificity for the detection of abnormal DNA in solid tissues and body fluids, as well as a wide range of metabolites and signalling molecules and immune system responses to drug therapies. Technological innovation in pathology appears to be accelerating the paradigm shift to precision or stratified medicine, an approach that takes into account individual variability in genes, environment, and lifestyle for each person.
A plethora of other technological advances are impacting on the pathology laboratory, including liquid and gas chromatography and plasma mass spectrometry, conventional and next-generation sequencing, point-of-care testing (POCT) and “lab-on-chip” devices prompted by miniaturization of molecular assay steps, biochips and microfluidics, and digital pathology systems which allow the scanning, imaging, and storage of histological slide data for analysis by pathologists.
The adoption of these emerging technologies across Europe, however, is varied and this is a fundamental challenge for pathology. The impact of new technologies on the hospital in the future will be difficult to predict but tough to ignore in terms of investment decisions. Technology has provided pathology with a unifying narrative and the vision of the laboratory as an aggregate of preventative, diagnostic, predictive, prognostic, and interpretative roles will become key to the development of the future hospital.
Some of the reasons for varied adoption or “technology diffusion” include: length of time the technology has been available; a hospital’s culture of embracing innovation (e.g. a large teaching hospital may find it easier to adopt new technologies because a translational research culture pervades); at national level there could be incentives and a supportive infrastructure to speed up the rate of adoption – or conversely nothing at all; it could be that some hospitals are simply better at measuring the impact of technology adoption in terms of patient health outcomes; in the case of POCT diagnostics, it is often the case that costs are accrued in a different area from the gains. The Review on Antimicrobial Resistance (2015) provides an example where a primary care facility may invest in a diagnostic device that could reduce the number of hospital admissions. While this is desirable, the costs saved are not only hard to quantify, but the money saved might not be passed on to the facility even though it has paid for the test.
Pathology departments are increasingly presented with opportunities to form translational research networks within hospitals, universities and biomedical research centres, and with industry. Several laboratories do not have the organizational resilience to translate research technologies to a clinical environment. In turn, this may prompt the need for new workforce capabilities aligned to the most desired patient outcomes within each European country.
As an endpoint, there is growing evidence to support the emergence of “population health systems” as a means of meeting future health care needs. A population health system is defined by the American-based Institute for Healthcare Improvement (2015) as a framework for improving patient experience, improving the health of populations, and reducing the costs of health care. Approaches to population health have long been enshrined in many tax-financed health care systems, forming the basis of the purchaser/provider split in the United Kingdom, Italy and some other countries since the 1990s, and in Scandinavian countries where health care is organized by local government, but are now gaining increased traction in other parts of Europe, as identified by the United Kingdom King’s Fund (Reference Alderwick, Ham and BuckAlderwick, Ham & Buck, 2015). The Kaiser Permanente model in America is often seen as a prime innovator in this regard. Making the shift towards effective population health commissioning will require collaboration across a range of sectors and wider communities and may intensify further change for the pathology laboratory in the hospital of the future.
Box 10.1 Case Study – Genomics England and the 100 000 Genomes Project
Context
Whole genome sequencing technology is sufficiently advanced to rapidly provide vast amounts of information on the nature of diseases and predisposing factors.
The technology is likely to impact on the delivery of a wide range of health care services, from inherited diseases, through infections, to cancer.
Challenges
By 2017 to sequence 100 000 genomes from NHS patients with rare diseases (and their families) and those with cancer.
To link genome sequences with high quality clinical and pathological information.
To accelerate the availability and uptake of advanced genomic practice into the NHS through better diagnostics, devices, and treatments.
To improve public understanding and support for genomic medicine.
Responses
Genomics England Limited created to drive the project, to inform training, and to develop partnerships with industry.
Eleven Genomics Medicine Centres created in 2014/15 with the remit to deliver against a specification and under strict performance management.
Achievements to date
Detailed protocols with research standards for the identification and recruitment of patients and families, sample collection and processing, and the validation of results and feedback of information to participants.
NHS Genomic Medicine Centres have developed local partnerships with the public, patients, and a range of local NHS organizations and universities.
Laboratory processes underpinned by an external quality assurance scheme.
The information technology required to support this complex process has been developed and implemented locally so that data collection is efficient and comprehensive. Data are transmitted securely to a central data hub.
Recruitment of patients and families to the rare diseases pathway started in April 2015.
Recruitment of patients to the cancer pathway began in September 2015.
Genomics England Clinical Interpretation Partnerships have been created as topic-specific groups of clinicians and researchers from universities and the NHS to analyse the data from the project. These will be integral to helping front-line clinicians and pathologists formulate the genomic data useful for managing patients in the context of personalized medicine.
Where does pathology sit within wider hospital activity?
Pathology is the largest diagnostic service in hospitals as measured by the number of requests it responds to annually, in expenditure, and in the proportion of clinical decisions it affects. For example, in the United Kingdom over 50% of biochemical tests are related to chronic disease management and pathology is involved in 70% of all diagnoses made in the NHS (Right Reference CareCare, 2011). Pathology is part of the clinical governance of public hospitals and the wider health system, playing an important role in monitoring and managing disease, infectious agents, and public health.
The development of subspecialties in pathology is well developed to meet the needs of patients: cytopathology, dermatopathology, chemical pathology, haematology, medical microbiology, virology, endocrine pathology, forensic pathology, immunology, cytogenetics, blood transfusion, neuropathology, ophthalmic pathology, to name but a few. However, these subspecialties are not uniform across Europe.
Depending on the urgency of tests, pathology investigations can take place in what are often termed “hot” or “cold” laboratories. Hot laboratories process pathology tests requiring a fast turnaround and clinical support. Cold laboratories process less-urgent high volumes of routine tests. Because there is less urgency to receive the results of these tests, cold laboratories can be located further away from the patient. As the technical complexity of test methods increases, so does the complexity of reporting.
Pathology services are closely integrated with other clinical services, to support patient care by providing information and expertise to facilitate diagnosis and treatment decision-making. This is particularly true with cancer, a disease process whose complexity is increasingly recognized, with a detailed understanding of the pathological characteristics essential for targeted treatment. The complex pathway undertaken by what might appear superficially to be the simple process of taking a tissue biopsy is set out graphically in an illustrated web page: http://www.journeyofatissuebiopsy.com
Adjacency to clinical teams is important if pathology is to be integrated as a valued “companion diagnostic” and to move away from having a passive service role to taking on an active one. Adjacency to molecular diagnostic centres will also become important in order to benefit from genomic (gene expression), proteomic (protein expression) and metabolomic (metabolite profile) data and to hasten the shift to personalized medicine.
Pathologists are core members of MDTs and provide essential inputs for patient management. Laboratories are used to working across primary and secondary care organizations and will often serve several secondary and tertiary care providers. Providers of pathology services in public hospitals also play a leading role in the education and training of pathologists, clinical scientists and researchers. They are increasingly required to provide specialist input for translational research including involvement with clinical trials and evaluation of new technologies.
The wider and extended role of pathology is demonstrated by the range of other clinical services provided, which includes:
specialist information and advice to health care professionals in primary and secondary care as well as public health
mandatory surveillance of disease
infection prevention and control
guidance and advice, quality assurance and support for POCT in a range of hospital settings (e.g. outpatient clinics)
specialist advice on blood transfusion
mortuary services, including post-mortem examinations.
Box 10.2 Case Study – a national framework for quality assurance in cellular pathology
The Irish National Cancer Control Programme
Context
Irish population = 4.5 million.
23 000 new cases of cancer annually.
7500 cancer-related deaths annually.
Challenges
Projected doubling of new cases by 2020.
High-profile cancer misdiagnosis cases in 2007 and 2008.
National histopathology workload increasing each year.
No formal measures to assure the public that pathologists practise to the highest international standards.
No national standards or benchmarks for key aspects of diagnostic service.
Responses
Development of a National Quality Improvement Programme within each Irish pathology department to review performance routinely and drive improvement against intelligent targets.
Programme initiated in 2008 with strong collaborative commitment from Irish Health Service Executive Quality Improvement, Service Management and Information and Communication Technology Divisions, National Cancer Control Programme, Independent Hospitals Association of Ireland, Department of Health and Faculty of Pathology, Royal College of Physicians of Ireland.
Achievements to date
A unique national programme across: 27 public and 7 private laboratories; 8 different laboratory information systems; and small and large hospitals with different levels of resourcing.
Robust clinical governance including monitoring and key indicator reviews.
Development of a central repository National Quality Assurance Intelligence System for Histopathology.
Collection of national data for histopathology which has never before collected on this scale.
Confidence in the data to understand in real time workload and extent of quality activities.
Ability to set national targets based on accurate and locally owned data.
How does pathology link with services located outside the hospital?
The interface between pathology and services located outside the hospital is well established but may not be well understood. The health care services based outside hospitals are described as primary and community care sectors, which lie between self-care and hospital care. Hospital pathology has a long history of working with outside services (i.e. primary care doctor practices and health clinics).
In Europe primary care differs considerably and several categories of organization exist. Reference MeadsMeads (2009) provides a valuable typology of primary care organizations in Europe. To add to the complexity, European countries will have different arrangements for registration with a primary care doctor or GP. This may be financially encouraged, compulsory, voluntary or free.
Pathology is a touch point across the patient’s lifecycle from pre-natal to post-mortem. The diagram below illustrates where pathology sits regarding screening, diagnostic, and monitoring functions.
Laboratories are often located in or near hospitals to meet demand for a 24/7 service. Many hospital departments are highly pathology-dependent and need to respond rapidly to the clinical needs of busy emergency medicine departments and intensive care units. This means that extensive networks of transport, IT and management links between laboratories have evolved outside the hospital to provide quick turnaround times for tests and equitable access to services over defined geographical areas.
There is also the wider reach of pathology services and diagnostic products into local populations. “Smart pathology” is emerging in the
form of new-generation POCT devices. The availability and use of these have steadily increased in Europe. They have been used in primary care, diabetic and sexual health clinics, and care homes for over 40 years, but are now being assimilated into high street retail outlets as well as the home. The rapid test turnaround time provided by POCT potentially allows for accelerated identification and classification of patients into high-risk and low-risk groups (Reference Larsson, Greig-Pylypczuk and HuismanLarsson, Greig-Pylypczuk & Huisman, 2015). There are, however, regulatory and quality assurance challenges which need to be overcome. The proliferation of so many additional users and devices in operation make the maintenance of acceptable quality levels problematic (Reference St John and PriceSt John & Price, 2014).
The future relationship between hospital pathology and primary care needs to be shaped by value expectations and whether value of service can be demonstrated through improved patient outcomes and managed costs. Insights could also be gleaned as to how a laboratory could better manage demand on its services and how this might benefit the local health community. Some examples could include the use of data for comparing testing rates to emergency admissions, number of tests requested, length of hospital stay, and cost of emergency readmissions for relevant conditions.
An example of this type of work was the INvestigation of ThE Root Causes of Excessive RepliCatE Pathology Testing (INTERCEPT) study, which involved over 115 000 patients from North Staffordshire, England, and aimed to reduce the burden of unnecessary pathology requesting. It used HbA1 c testing (a test for monitoring blood sugar control in people with diabetes) as a model by assessing adherence to national guidelines and recommendations for retesting intervals. Results from the study found over half of key blood test requests from doctors for patients with diabetes were inappropriate and that guidance on monitoring diabetes patients was not being followed by the majority of primary care and hospital doctors. Further work by this research team found major incentives to establish systems that provided timely HbA1 c tests in terms of fewer diabetes-related emergency admissions per 1000 patients, fewer hospital bed days, and reduced costs of emergency admissions for diabetes-related illnesses (Reference DriskellDriskell et al., 2012).
The key message for health commissioners and policy-makers is that primary care engagement with pathology professionals and wider use of this type of data can change requesting behaviour and produce better patient outcomes.
The pathology workforce
Pathology is a combination of medically trained pathologists, clinical scientists and biomedical scientists together with essential support from staff occupying a wide range of laboratory roles. Each European country uses different pathology specialty taxonomies. The European Union of Medical Specialists identifies 43 specialist sections, with the sections relevant to pathology including clinical genetics, infectious diseases, laboratory medicine, medical biopathology, medical microbiology, and pathology. The workforce is therefore extensive and heterogeneous in its composition.
Across Europe demand for diagnostic services continues to rise year on year both in terms of the number of samples and the increasing complexity of test requests. This puts considerable pressure on the workforce as the emergence of new tests steadily drives up case volume. Europe’s ageing population and the increased incidence of cancer, chronic diseases, and other co-morbidities continue to add pressure.
Advances made in molecular-based diagnostics offer new approaches and the number of variants generated that have as yet unknown medical significance will require clinical interpretative support. Since the time of Hippocrates all medicine has been “personalized” at the point of diagnosis and treatment, but genomics and molecular-based diagnostics bring the potential for personalized prevention strategies based on the inherent likelihood of future disease for each individual. Also these new technologies will require the monitoring of the effects of ever more complex individually tailored drug treatments. Technologies that are part of molecular diagnostics are far reaching and rapidly being developed for genetic testing, infectious diseases testing, blood screening, oncology testing, cardiovascular testing, and others. Continued growth is expected. It is anticipated that a constant stream of test kits for the newest molecular targets will become commercially available requiring pathology staff to expand their understanding of these techniques to provide the services and interpret the results. Far from being replaced by new technology, it seems likely that demand for most “traditional” pathology tests will increase due to increased uptake of molecular-based diagnostics. This must be borne in mind when considering workforce requirements.
Pathologists now perform complex investigations to determine the phenotype, prognosis and likely response to treatment of a variety of diseases. The potential clinical significance of these data frequently cannot be encompassed in simple reports but require detailed interpretation and simultaneous communication to clinicians and to patients in order that appropriate management strategies might be formulated, agreed, and reviewed as response to treatment becomes apparent. There will be a need for pathologists to provide more interpretative and advisory services directly to patients as they obtain the right to access their own results directly. Pathologists increasingly find themselves making significant and important contributions as to how diagnostic testing can improve the whole patient pathway. This may include guidance, explanation, and interpretation provided to other health care professionals less able to deal with the complexity of modern diagnostic medicine.
Chemical pathologists, as a direct consequence of the increasing prevalence of diabetes, obesity, and lipid disorders, are pivotally involved in the provision of direct specialist patient care. This will inevitably lead to more involvement in community provision of pathology services and the support of patients to reduce morbidity. As microbiologists, haematologists, and biochemists become more clinically involved in providing direct patient-facing care, they have less time available to provide traditional laboratory oversight. The oversight of laboratory services is intrinsically linked to the quality of the service provided and to patient outcomes and so the importance of external quality assurance monitoring will increase.
The increasing use and dependency on POCT will continue to expand, not just in primary and secondary care, but also in the high street and in patients’ homes. There will be vital input required from pathology professionals to ensure that the technical aspects of such POCT is carried out to an adequate quality-assured standard in the correct clinical context.
Pathologists make significant contributions to research, both directly via their own research activity, but also by providing essential and important collaboration and diagnostic support to many other studies and trials. However, a worrying trend in some European countries is the demise of clinical research roles in pathology.
Scientific and medical staffing levels in pathology services are declining in most countries and a detailed analysis of the workforce crisis in the United Kingdom in relation to cancer services is highlighted in a Nuffield Trust report (Reference Imison, Castle-Clarke and WatsonImison, Castle-Clarke & Watson, 2016) and in a Cancer Research UK review (Reference BainbridgeBainbridge et al., 2016). One possible solution is improved training and broader roles for scientific staff traditionally not involved in detailed microscopic cancer diagnosis, as illustrated in the case study in Box 10.3.
Biomedical Scientist Histopathology Reporting Pilot in the United Kingdom
Context.
Clinical scientists are an accepted facet of clinical provision in some pathology disciplines.
In 2011 the NHS Information Centre found only 17 consultant clinical scientists in cytology and histopathology in the United Kingdom.
Some extended roles for scientists already exist in cytology, macroscopic dissection, and molecular pathology.
There is no formal clinical scientist training programme in cellular pathology.
Challenges
Projected increase in new cases of cancer.
Increased quality-assurance scrutiny and national key performance indicators.
Inability to fill consultant vacancies in many parts of the country.
Large backlogs of patients’ biopsies and resections awaiting reporting or being outsourced.
Career opportunities limited for scientists in cellular pathology.
Predicted reduction in demand for cervical cytology as a primary screening modality.
Responses
Development of an RCPath-led nationwide pilot of new ways of working in cellular pathology.
Participants trained to report cellular pathology in clinical context.
High volume, low complexity and low litigation areas of practice initially chosen to prove concept.
Pilot participants recruited in 2012, 2013 and 2014.
Curriculum and assessment tools developed with RCPath approval and strong collaboration with the Institute of Biomedical Scientists.
Achievements to date
An innovative national training programme across 37 NHS hospitals.
Robust educational standards and clinical assessments.
New Conjoint Board established with the Institute of Biomedical Science to move the pilot onto a permanent footing.
For the future hospital
Expansion of areas of reporting practice planned with the introduction of new curricula and training programmes.
Expansion of the recruitment into cellular pathology reporting to wider health care scientist population.
Formal clinical scientist training programme in cellular pathology.
Barriers to optimal pathology services
Barriers impeding the delivery of an efficient and effective pathology service include those related to service configuration, demand management, workforce, finance, quality, attitude, IT, and innovation adoption. These are discussed in turn.
Service configuration barriers
In some European countries there are too many laboratories carrying out specialist tests on too small a scale. Reconfiguration can be seen as a way to optimize pathology and to attain economies. However, consolidation, such as joint ventures and mergers, must be compliant with national and European competition law, which can constrain reorganization, as well as the undesirability of creating commercial monopolies that can lead to higher costs, worse performance, and reduced innovation.
The impetus for transformation can be slowed or blocked because pathology is not high enough on hospitals’ priority lists. Many hospitals are evaluating options to develop new models of care within social, community, primary, and secondary care and this may push pathology modernization further down the chain of importance.
Demand management barriers
Most laboratories across Europe have experienced significant increases in workload year on year and the capacity of a service to manage demand is stretched, especially without a commensurate rise in staffing levels. Workload, measured in terms of crude sample numbers or test requests, is increasing and this probably belies actual workload because greater sophistication of diagnosis is now needed.
For example, increasing numbers of cases now require consensus reporting and referral for specialist opinion, demonstrating increasing sophistication of diagnostic processes and an increasingly risk-averse culture. Equally, more objective assessments are now required, whether it is a lead to provide reproducible assessments that determine patient treatment (e.g. quantitative immuno-histo-chemistry results which act as a threshold for breast cancer oncotherapy).
Where pathology is excluded from strategic planning processes and investment decisions, there is a risk that there will be unexpected, unplanned, and unfunded demands on those pathology services in the future.
Increased expectations will contribute to demand pressures in the following areas: providing ongoing clinical advice to doctors in training and primary care doctors, direct interpretative and advisory liaison work with patients who can access their test results directly, and provision of direct specialist outpatient care in diabetes, obesity, lipid disorders, and metabolic diseases.
Medical microbiology has seen an increased requirement for ward-based consultation with patients with suspected or proven infection, as a means to facilitate earlier discharge from hospital. Increasing antimicrobial resistance has placed greater emphasis on antimicrobial stewardship, with pathologists working alongside pharmacists specializing in antimicrobials.
There is a need for payment systems to take account of these rapid changes, with regular revisions that recognize new ways of delivering care. The regular reviews of the system for paying providers in Germany offers such an example.
Workforce barriers
Wider training issues, such as the trend in some countries to expand general medical training before specialization, could lead to a shorter time for pathologists to acquire specialist competencies. Recruitment to particular pathology specialties has been problematic. Outsourcing tests or using locum staff may alleviate workload but this only represents a short-term and expensive solution. There is uncertain capability to undertake some emerging techniques and technologies. The ability of pathologists, for example, to understand the disease phenotype (the detailed characteristics of the patient) is essential for interpretation of the current explosion in “-omics” data, i.e. genomics, proteomics, metabolomics, and transcriptomics.
Financial barriers
Traditionally, diagnostic tests in pathology have seemed cheaper than, for example, the costs of imaging. However, with many pathology services increasing their repertoire to include molecular testing, costs are increasing. A test costing €1000 could be perceived as being expensive but this has to be seen in context, such as whether the test is used to determine the use of a drug treatment which may cost more than 10 times as much. Unfortunately there can be a focus on the unit cost of pathology rather than looking more holistically at the “downstream” value for money that pathology contributes to the whole health care economy.
Establishing a transparent tariff for pathology tests could be beneficial, as is the case in many countries, such as Germany. Having tariff transparency would enable business cases for service transformation to be built up more easily.
Quality barriers
Many pre- and post-laboratory processes remain outside laboratory control, even though they impact significantly on the value of the service. End-to-end quality depends on others (e.g. requesting clinicians) over whom pathology has less control. The quality of the clinical pathology service will be impacted where there are areas of differential influence and control.
Appropriate ordering and commissioning of relevant laboratory tests and having timely access to the tests and test results are central to the provision of quality care for patients and patient flows through the hospital system. There is considerable variability in awareness and understanding, which leads to suboptimal and inappropriate use.
ISO 15189 accreditation may have value in assessment of laboratory quality management systems but is highly expensive to maintain and is entirely focused on processes within the laboratory and not on the end-to-end pathology contribution to health care.
Attitudinal barriers
Van Krieken, President of the European Society of Pathology, identified a lack of collaboration between pathologists and other stakeholders such as the pharmaceutical sector. His idea is to move towards a system in which tests and drugs are integrated, so that payment for a drug includes all necessary testing (Reference van Kriekenvan Krieken, 2015).
It is frequently observed that pathologists themselves need to take on more of a clinical leadership role and move out of the shadows. Risk-averse over-requesting of tests can prevail due to perceived threats of medico-legal liability and a monetary incentive may exist for over-requesting in some systems.
If testing is perceived as a cost-free service as far as requestors are concerned, there is little incentive to avoid waste and duplication. The most effective method of managing demand and promoting new technology is to ensure appropriate recovery of cost to the laboratory budget from other clinical budgets.
IT barriers
Within many European countries a wide range of IT systems are in use and this creates problems of interoperability between service users and pathology. In radiology the Digital Imaging and Communications in Medicine (DICOM) standard has achieved a near-universal level of acceptance among medical imaging equipment vendors and health care IT organizations but such a standard does not yet exist in pathology.
The lack of end-to-end IT connectivity in pathology limits the opportunity to achieve effective communications between laboratories and those ordering tests, as well as decision support, both of which minimize inappropriate or unnecessary repeat testing. There is a widely held perception that results data are not being fully leveraged by pathology service users and providers, and this is a key obstacle to cost and service improvement.
Innovation adoption barriers
At a national level delays in approval processes can constrain innovation. At a local level many test sites will be required by ISO 15189 to perform their own evaluation of a new test and duplicate many of the assurances already fulfilled by the test developer.
Aggressive national pathology cost saving plans may discourage adoption of new techniques. A complex cost–benefit relationship often underpins decisions to use new devices. Point-of-care devices are a good illustration and highlight how costs and benefits are often accrued in different areas. A primary care group may have funded a diagnostic device and reduced the need for patient hospital visits but may not receive the benefit of saving money for the health system because of opaque reimbursement mechanisms.
Investment in innovation for some pathology specialties has been limited. For example, many drug companies have no commercial interest in the development of rapid diagnostics for determining antibiotic sensitivity because of low commercial returns. The uptake and adoption of diagnostic tests across Europe shows significant variation. For example, C-reactive protein (CRP) tests have been used for some time in the Netherlands and Scandinavia to indicate whether an infection is bacterial or viral, and these countries have some of the lowest rates of prescribing antibiotics in Europe (Review on Antimicrobial Resistance, 2015).
In recent years there has been growing interest in using more accurate, efficient and reliable technologies such as mass spectrometry. Despite the important scientific advantages of such technologies, many clinical diagnostics services have continued to use traditional immunoassays, facing barriers such as the need for investment and expertise in mass spectrometry.
Obsolete but not yet abandoned
Obsolete ways of working include: laboratory standard operating procedures existing in isolation from patient pathways, single-handed pathologists, and old methods and out-of-date technologies.

Figure 10.3 Quadrant highlighting pathology trajectories
Declining but not yet abandoned
Some aspects of hospital laboratory medicine are in decline but have not yet been abandoned. In some instances this is because there is a hope that the importance of these activities will be recognized. Notably, research capacity in pathology is in a steady decline and academic pathology is small scale and disjointed in all but a few major teaching centres where huge efforts are being made to keep up this aspect of the service.
In the United Kingdom there was such widespread concern about the loss of research capacity in 2015 that the National Cancer Research Institute, together with the ECMC Pathology Network Group, funded the Cellular Molecular Pathology initiative (CM-Path) – a five-year project which was awarded £635 k. It aims to reinvigorate pathology research by building the change needed to support academic cellular molecular pathology. A report on the Experimental Cancer Medicine Centre Initiative (2015) gives more details.
Also, most pathology services are making great efforts to retain the interpretive and clinical advice aspects they provide, which is threatened by low-cost, dumbed-down “results-only services” which sacrifice patient-centred care and close working relationships between pathologists and clinical requestors. Importantly, hospital autopsies are in decline all over Europe (Box 10.4).
Box 10.4 Case Study – decline of consented autopsy following hospital death in Europe
Context
Consented autopsy rates have fallen significantly in Europe over the past half century to the verge of extinction (Reference Turnbull, Osborn and NicholasTurnbull, Osborn & Nicholas, 2015).
The benefits of autopsy are established and include: clinical audit, patient safety, public health in a time of global antibiotic resistance, epidemiology, research, education, improved mortality statistics, improved diagnostics, improved resource allocation, comfort and explanation to grieving families.
The priority of the autopsy in modern health and social care is highlighted by the Francis report and in the United Kingdom will be crucial to the work of Medical Examiners of the Cause of Death due that was due to be implemented in England in 2018.
Challenges
The main reasons for the decline are: perceived difficulties in obtaining consent; a limited role given current diagnostics (“autopsy is pointless in modern medicine – we know the diagnosis”); and religious objection.
In some countries legislation is thought to have had an impact on hospital autopsies, such as the Human Tissue Act in the United Kingdom.
A change in attitude is required so that autopsy is considered an altruistic act similar to organ donation.
Responses
Most religions contain no objection to autopsy and most families would consent to autopsy if appropriately asked.
The number of diagnostic discrepancies would decline with an increase in autopsies.
Diagnostic discrepancies may be due to co-morbidities or atypical clinical presentation.
Despite technological progress, autopsy still has an important role in the assessment and improvement of the quality of surgical practice.
Achievements to date
The European Critical Care Foundation (ECCF) held a conference in 2015 on the decline of the hospital autopsy to raise awareness among health care professionals.
The idea to establish a pan-European anonymized autopsy database (“Europsy”) was proposed at the ECCF meeting.
For the future hospital
Autopsy should be offered to families across Europe upon the death of a relative to demonstrate willingness to discuss the patient’s last episodes of care.
Pathologists need to clarify and simplify the consent process to design a simple, yet effective, autopsy consent form.
Alternative autopsies such as digital autopsies could be encouraged but their limitations should be understood and they are currently expensive and do not allow tissue to be obtained for in-depth diagnostic and research purposes.
Medical research requires accurate causes of death. Autopsy should be used as a gold standard end-point for any deaths occurring in clinical trials.
Specialist hospital professionals could be trained in consent, such as a pathology liaison nurse whose role would be to gain consent, to provide feedback to clinicians and to families, and to teach hospital staff about death.
Teaching opportunities should be exploited so that students and junior doctors gain greater exposure to autopsy practice.
Existing but not widely implemented
Exciting new ways of providing pathology testing are not always implemented widely. For example, expansion of POCT and self-testing into the high street and homes has begun but in some instances is limited by patient acceptability (for a more detailed exposition see Reference Larsson, Greig-Pylypczuk and HuismanLarsson, Greig-Pylypczuk & Huisman, 2015). There are many other barriers to take-up of these devices, including the test devices themselves, patients’ use of and interaction with the devices, providers’ understanding of their uses, and the health systems in which they are used. Successful uptake usually requires integration of knowledge at these levels, which in turn can lead to trust and confidence.
The implementation of digital autopsies is limited by the diagnostic limitations of the technique. Standardization of units of measurement, reference ranges, coding and methods is required to enable results data to be of benefit in monitoring long-term conditions and in disease prevention, and implementation is limited by failure to implement national developments such as the National Laboratory Medicine Category in the United Kingdom.
Pioneering and aspirational
Some aspects of pathology service provision are envisioned but not yet in place. A key reason for this may be the traditionally slow rate of uptake of new technologies in pathology. The following technological innovations are discussed:
Wearables
Wearables are devices with sensors that monitor physiology. They can be integrated into devices such as smart phones, fitness bands, and clothing to track health and fitness. It is conceivable that these devices are able to generate self-monitored health data which could then be streamed directly into cloud-based data repositories or patient electronic health records. From here, general practitioners and hospital clinicians could access the data. However, several questions arise, including whether these devices are fit-for-purpose in bypassing an initial patient diagnosis and whether they can be used to triage a problem and direct the patient to relevant specialists. The accompanying growth of related apps could also facilitate transfer of data across different platforms and devices and lead to greater interoperability but it is too early to know whether patients would actually want this to happen. It also raises some regulatory issues as the devices would have to be cleared to use the same biomarkers which are used in clinical laboratory tests.
The challenge for pathologists and laboratory managers is that if wearables become mainstream, strategies will need to be developed for data collection, understanding what utility these data will have and how to manage such data in conjunction with conventional laboratory test data.
Biosensor point-of-care devices
A biosensor is a compact analytical device that detects, records, and transmits information regarding a physiological change or process. The use of biosensors is well established in the management of chronic illnesses, such as blood glucose monitoring in diabetes and cholesterol monitoring in cardiovascular disorders. Biosensors have also shown potential for in vivo sensing of disease-specific biomarkers such as cancer. Here, sensors with nanoscale dimensions have been developed for effective diagnostics purposes (Reference HasanHasan et al., 2014). Biosensors have many advantages: they are easy to use and yield fast results; there is no need to use labelled reagents; the cost per test ratio is low (although initial investment in the device is needed); and only a small sample is required. Challenges which need to be overcome focus predominantly on sensor accuracy and their minimum detectable levels. Additionally, it could be argued that some biosensors are “pseudo-portable” because their detection platform relies on bulky fluidic and detection systems.
Undoubtedly the next-generation whole-cell biosensors will see continued miniaturization of components, improved computing power, enhanced amplification capacity, and applications made further afield. The migration of some pathology tests from laboratories to point-of-care devices will continue and it is hoped that concerns about quality assurance and reliability, and their integration into a locally managed pathology network, will be fully addressed.
The promise of using POCT devices as an effective diagnostic in other contexts such as general practice is under review in many European countries. In the United Kingdom the National Institute for Health and Care Excellence (2014) issued draft guidance which recommended that GPs should consider using a POCT (CRP) to help decide whether patients presenting with mild pneumonia need antibiotics. A narrative review of primary care POCT and antibacterial use in respiratory tract infection was undertaken by Reference CookeCooke et al. (2015). The researchers drew attention to a survey of Dutch general practitioners who reported that the most common POCTs currently used by family physicians were: blood glucose (96%); urine leucocytes or nitrite (96%); urine pregnancy (94%); haemoglobin (58%); and CRP (48%). The most commonly desired POCTs were: D-dimer (70%); troponin (65%); brain natriuretic peptide (BNP) (62%); chlamydia (60%); and International Normalized Ratio (INR) (54%). In terms of wider scalability for POCT devices, agreed protocols would have to be in place for data sharing across connected diagnostic networks within constituent countries as well as across Europe.
New technologies in pathology are sometimes heralded as game changers that will bring significant benefits to patients and providers alike. However, caution is needed over claims made by new technologies. The Theranos company is a case in point. Theranos was an American company founded in 2003 which successfully raised capital to streamline and standardize blood tests by creating a hand-held device using a few drops of blood obtained via a finger-stick “nanotainer” vial. It developed its own proprietary analyser to test blood samples. However, there were allegations against Theranos about discrepancies between a number of their specific blood tests when compared with traditional quality-assured methods. This resulted in a formal complaint to US regulators, which led to a finding that several clinical standards had been violated. A review of Theranos’ systems, processes, and procedures resulted in Ms Holmes being charged by the Securities and Exchange Commission with widespread fraud, accusing her of exaggerating – even lying – about her technology while raising $700 million from investors said to include some of the world’s richest people (New York Times, March and May 2018). There is a cautionary tale in the adoption of new technologies in pathology service. It is essential that the clinician is at the centre of technological adoption in the interests of patient safety and quality of care.
Conclusion
The tree of medicine diagram below provides a reminder of the centrality of pathology in medicine, as the trunk of the tree that links all aspects together is pathology.
Pathology in European hospitals is at a crossroads, with the future contingent on a willingness to address the barriers discussed in this chapter. There are many opportunities for pathologists to play a central clinical role. Despite operating under unrelenting fiscal constraints in some countries, pathology is entering into the “genome era” and pathologists must acquire and demonstrate visionary leadership.

Figure 10.4 The tree of medicine (date of publication unknown)
Pathology services tend to be ignored by policy-makers and managers, and a key challenge will be to demonstrate how high quality pathology provision improves accuracy of diagnosis and effectiveness of monitoring or treatment, so creating better patient health outcomes. Quality in pathology reduces patient pathway costs as well as providing key health care data and impacting on all other health care interactions. The redesign of pathology so that it becomes part of an integrated patient pathway should be explored and communicated so that a clear demonstration of its value can be evidenced. This would enable pathology to be delivered where it is required while operating within an integrated quality framework (Reference MyersMyers, 2014).
Almost every aspect of society today has been shaped by technological developments. Take the nature of the modern state. The historian Philip Bobbitt describes how the introduction of gunpowder to Europe rendered the medieval city states, protected by high walls, obsolete. Gutenberg’s invention of the printing press, allowing for the cheap distribution of information to the masses, paved the way for the Reformation and later for revolutions. The discovery of magnetism, and thus the compass, made it possible to establish global networks, enabling exchange of people and ideas and, ultimately, the system of international trade that prevails today. The invention of the steam engine, powering both railways and mines, paved the way for the industrial revolution and, with it, the growth of major cities. These examples illustrate how technological advances have created huge societal changes that rippled out into further cycles of innovation, driving the shift from local feudalism to a global post-industrial society.
Health care has similarly been influenced by technological change. As described in the first chapter, the modern hospital owes its origins to the need to concentrate resources around laboratories, operating theatres, and X-ray facilities. Safe anaesthetics, antibiotics, and the concept of asepsis changed hospitals from places where patients increased their risk of dying simply by entering to ones that could cure or, if this was not possible, alleviate symptoms. Yet, as also noted in that chapter, many of the assumptions that underlie the concept of the modern hospital are now being challenged. Numerous examples throughout this book show how technological advances are changing the way that health care is provided. In some cases these advances are specific to health care, such as desktop kits that take over many of the functions once reserved for the laboratory, or mobile monitoring systems, such as those that can track physiological changes in patients as they go about their everyday life. For example, it is now possible to attach an ultrasound probe to a smart phone that will allow a health professional to look inside the body of their patient even in the remotest of areas. Patients can also have their chronic conditions managed without the need to regularly travel to hospital appointments, as in the case of COPD where specialist expertise can be obtained at a distance.
Other technological advances are generic, such as advances in communications technology. The smart phone that most people carry has the computing power of a supercomputer of the 1960s. Information and images can be transmitted rapidly between teams of health professionals, ensuring that all have up-to-date information on the patient they are managing and giving access to specialist advice from experts across the world and in future to artificial intelligence to support image analysis and decision support. In some cases, in future, sophisticated image analysis software will outperform skilled clinicians.
These developments have several characteristics. First, most were not anticipated or, if they were, the consequences were often very different from what was first predicted. For example, while the discovery of insulin had, as expected, a transformational effect on the survival of young people with diabetes, it took many years before the long-term complications of diabetes, and with them the need for new models of care, became apparent. The same was true of the introduction of antiretrovirals for HIV. It is only now that the long-term complications of infection with the virus and the accompanying immunosuppression, as well as the side-effects of the medicines, are being recognized, such as increased risks of cardiovascular disease and certain cancers. Fleming’s discovery of penicillin transformed the management of many common infections but within a few years the problems of antimicrobial resistance were being recognized.
Second, many have required significant changes in ways of working. The survival of patients with noncommunicable diseases has given rise to the challenges of multimorbidity, which in turn has stimulated the creation of MDT working. Advances in diagnostics and treatment have allowed many patients who once would have had to attend hospital to be managed in the community. Many technologies require the development of staff with new skills and some have led to the emergence of new disciplines – for example, interventional radiology and cardiology. Some have allowed tasks previously undertaken by highly trained professionals to be delegated to other staff and in some cases to the patient or their carers, for example monitoring blood sugar for diabetes or clotting to manage anti-coagulation. It is worth noting that much of this change has been in advance of, rather than in response to, changes in policy to payment systems and that policy-makers and payers have often struggled to keep up with the pace of change. Regulations, payment systems, and directives can inhibit and support changes but they are only part of the story of how these technologies are adopted. There are also lessons about the way that poorly designed incentives can create over-adoption: such as the multiplication of cardiac facilities in Bulgaria due to very high profit margins that were unintentionally created by the payment system.
Third, while some of these changes have been transformational, their development and spread have generally been incremental. For example, new, safer, and more effective medicines in the same class provide clear benefits, but do not demand new models of care. Others are more disruptive, such as the earliest developments in minimally invasive surgery, the development of endoscopy, interventional radiology and angioplasty in some cases challenging established ideas about by whom and where care is provided. Another more structural example is stroke units, which have revolutionized stroke treatment over the last quarter of a century. They both improve survival and reduce long-term dependency. Moreover, the delivery of early supported discharge, which involves patient care and therapy in their own home following stroke, has been shown to shorten length of hospital stay and improve long-term recovery, thus challenging old treatment pathways.
The clear message from the history of technological advances is that they cannot be ignored. Just as in the past, they will continue to shape the nature of health care and, with it, the roles of those who provide it and the ways in which they work together. To enable the hospital to support these changes rather than obstruct them, attention will need to be given to thinking more creatively and strategically about the workforce, technology, design of buildings, and the wider system in which hospitals operate.
Hospitals will need to be designed in a way that is sufficiently flexible to adapt to these changing circumstances, both in their physical design and their organizational structure. A hospital built today will be unrecognizable to doctors and nurses from the early 20th century. Resistance to change is simply pointless. Yet, too often, it takes years to take full advantage of innovation. Health care often lags far behind developments in other sectors, illustrated when, in 2017, the computer system of large parts of the English NHS were paralysed by a ransomware attack that exploited systems using the obsolete Windows XP software. Nonetheless, health systems also demonstrate many remarkable examples of entrepreneurialism, with individual clinicians and their teams introducing innovative technology and ways of working, despite the system in which they work seemingly doing everything possible to obstruct them. The challenge, for health policy-makers, is how to encourage this entrepreneurialism in ways that maximize health gain, while not destabilizing the overall health system.
Preparing for the future
In the following section, we look briefly at some of the examples of innovation that reflect themes in earlier chapters and the opportunities and challenges that they pose for the hospital now and in the future.
We begin with the multidisciplinary team. As noted above, the growth of multimorbidity and the complexity of the responses to it, involving different groups of professionals, require completely new ways of working. A typical patient aged 75 or above may have five or six different conditions, each requiring long-term medication or other forms of therapy, not all of which may necessarily be compatible. Yet they may still be able to lead a normal life with appropriate input from different professionals. This requires a high level of organization, with seamless transmission of information. These patients are on a journey, and the challenge for the health system is to make it as smooth as possible. Unfortunately, in practice, it can be more like an exploration of an unknown land, moving from point to point almost at random, often getting lost in the process. Advances in technology can improve this process, in particular by ensuring the timely sharing of information. However, much more is needed. In particular, such teams can only operate in a culture characterized by collaboration, with flat hierarchies and mutual respect among all those involved. Creating these teams is not easy and requires deliberate work to develop and maintain them. Research in health care suggests that the appearance of teamwork may often disguise a lack of clear purpose, poorly defined membership, leadership problems, unhelpful hierarchical behaviours, and a lack of support for the team (Reference West, Markiewicz, Ferlie, Montgomery and PedersenWest & Markiewicz, 2016).
MDTs in cancer care involve coordinated working among different professionals, which is required to synchronize the complex array of interventions and frequent patient contacts. Oncology MDTs can include a broad range of health professionals with different skills including in diagnostics, oncology, pathology, radiology, surgery, nursing, and palliative care, who must work together and also alongside other professionals in psychology and psychiatry. Also professionals involved in new models of perioperative care, which emphasize improvement and consistency of outcomes for patients after surgery, are fundamentally multidisciplinary. Health professionals are drawn from a range of medical specialties, including anaesthesia, surgery, geriatric, and internal medicine, and should be led by those who can take a system-wide approach.
A related issue is the tension between generalists and specialists among health professionals. Unfortunately, in many health systems the specialist occupies a privileged position in the medical or nursing hierarchy, making it difficult to attract and retain generalists. Patients with multimorbidity will from time to time require highly specialized inputs. For example, a patient with diabetes, among other conditions, may need laser treatment on their retinas. This is a highly skilled task. They may also have kidney failure requiring dialysis, again a task requiring considerable expertise. But at the same time, they need someone who can take a holistic view of their health problems, ensuring that a treatment initiated for one problem does not exacerbate another. The growth of multimorbidity and polypharmacy as populations age presents a significant challenge to the model of narrow specialism. Patients increasingly fail to fit neatly into the way that medical specialisms have been organized.
As a consequence the fastest-growing area in hospital medicine in the USA has been in the specialism known as “hospitalists” (Reference Wachter and GoldmanWachter & Goldman, 2016). These are often internal medicine specialists (although they can be drawn from other disciplines) and are now appearing in paediatrics and other areas. Their role is to act as coordinators of patient care within the hospital and to co-manage cases with some specialties. Social complexity and difficulty in discharging patients as a result are also problematic and the hospitalist movement has been criticized for not paying sufficient attention to these issues (Reference GundermanGunderman, 2016). The chapter on frailty offers a similar model of a general physician with specialist skills for managing complexity, but shows the importance of services that can cross the boundary between the hospital and other types of care and address patients’ wider needs. Although this has been focused on older people, these issues of complexity are not confined to the old. The question of the optimal balance between specialist and generalist care has not been answered.
In countries where primary care is the main provider of care for chronic diseases, the increasing levels of demand and the large and growing body of scientific knowledge involved in managing chronic conditions mean that there is a need to help primary care doctors, nurses, and other clinicians in their work and in keeping up to date. Hospital specialists in areas such as endocrinology, respiratory medicine, nephrology, cardiology, rheumatology, etc., have a key role in supporting the management of conditions such as diabetes, heart failure, and asthma, overseeing the administration of complex treatments and providing feedback and help with activities such as quality improvement and process redesign. This may require new skills, different approaches to patient consultations, and a change in the relationship between hospitals, primary care, and patients. The key aspects of this include:
Rethinking the traditional outpatient model based on referral to a specialist.
Improving case management skills of health professionals to ensure that the patient’s problem is dealt with or that the patient is quickly referred to another professional who can deal with that problem.
Health professionals working proactively to identify risks for the patient and engaging with them to address these. Often these may require action to deal with non-medical problems in the patient’s life that are making compliance with treatment plans difficult.
Considering and developing strategies for population health and prevention. This will include specialists taking a more direct interest in these areas, including secondary prevention for their existing patients and more active involvement in health promotion for the wider population.
Specialists acting as consultants and overseers of networks of care and supporting other professionals. This means that the type of patient they deal with will often be more complex.
These challenges have led to a great deal of interest in the creation of various types of integrated care organizations that bring together primary and specialist care, and which potentially can deliver care that meets the characteristics described above. Many of these changes to the relationship between the hospital and its wider system will support integrated care but it is easy to underestimate the scale of the changes in work processes and operating models for hospitals and the staff who
work in them that the full development of these models will require. If integrated care systems can deliver on their promise of reducing the use of hospitals, then there are some major challenges as to how to reduce fixed costs if there are reductions in the use of hospital facilities.
The growth of specialism and the narrowing of many specialist fields mean that all but the largest hospitals will not be able to have the full range of expertise on site. The growth of digital technology means that laboratory and imaging expertise does not necessarily need to be in the same location, or even the same country, as the patient. The development of communications technology also offers the opportunity to spread expertise across distances. This can support the growth of specialist referral networks with escalation criteria and standardized protocols. These networks are increasingly common in cancer, neonatal care, neurosurgery, and many rare diseases where there is already a strong trend towards centralization because of a strong body of evidence that for certain types of care – particularly complex care, some types of surgery, and cancer care – higher volumes are associated with improved outcomes.
Referral networks are also found in high volume areas such as maternity services, where different parts of the network will have rules for accepting or transferring patients relating to the level of risk involved. Sometimes these may include retrieval services to ensure the safe transfer of critically ill patients. The organizational arrangements to allow for rapid transfer and return of patients need to be agreed across the network and properly managed or will be a cause of some tension.
The development of hospital networks run by groups such as Helios and Asklepios in Germany, and IHH, Apollo and Parkway in Asia, and which are also increasingly found in other European countries, partly reflects a growing idea that there are economies from both scale and standardization. Agreeing a common approach to a procedure, such as hip replacement, allows for procurement savings but also creates the potential for benchmarking and improvement across a wide network with managed processes to make this happen, as opposed to relying on hospitals joining such approaches voluntarily.
The growth of technology and a strong emphasis on efficiency have had the effect of shortening lengths of stay and increasing the intensity of work in hospitals. This trend will continue and will put increased demands on staff, facilities, and engineering and means that the proportion of beds run as critical or high dependency care is likely to rise.
A second effect has been to move work and specialists, who have been traditionally based in hospitals, to ambulatory settings, creating new ways of delivering care and requiring different approaches to giving specialist advice for inpatient care.
While advances in technology have brought many benefits, they have also created new challenges. One relates to the challenge of providing effective health care to people living in remote areas. As has been noted, the management of conditions such as myocardial infarction, gastrointestinal bleeding, stroke, and major trauma have been transformed by the introduction of new methods to intervene actively to tackle the fundamental problem, whether it be a blocked artery or catastrophic bleeding. Yet for this to be achieved, there is a need for rapid diagnosis, followed, equally rapidly, by definitive treatment. If these are delayed, the treatment is simply ineffective. Yet in some places, where the population density is low, it will never be possible to provide such definitive diagnosis and treatment sufficiently close to where people live. This will require new and imaginative solutions involving the training of multiskilled doctors and other clinical staff, technology for remote advice and support, and rapid transfer or retrieval services. Remote areas tend to be more explicit with their local population about the limits and capabilities of local services and what will happen in the event of a serious emergency than those in more populous areas.
There are challenges as well as opportunities from the increasing role played by information technology. There is a danger that feeding the system with data can take priority over interacting with the patient. Patients frequently complain that the health professional spent the encounter looking at a screen rather than at them. Health professionals complain that they spend so much time entering data that they are unable to engage in conversation with the patient. Yet in other sectors this challenge has been addressed. There are many new means of entering data, ranging from barcodes to the use of voice recognition software. Unfortunately, in the health sector these appear to be difficult to implement and are significantly under-exploited.
The way forward
We conclude with four recommendations. In producing this book, we have been struck by the lack of fora within which those working in hospitals, those responsible for their design and operation, and those responsible for the policy environment in which they operate can come together to exchange ideas. There are many innovative models of care around Europe but far too few have been evaluated and, where they have been, the findings are not easily available. There is now clear expectation that those responsible for introducing therapeutic innovations, such as new medicines or surgical procedures, should evaluate them and share the results. This is not the case with innovative models of care. There is a clear need to create mechanisms that would enable this to happen.
The second relates to the hospital workforce. The roles and responsibilities of health professionals have changed remarkably over the past few decades. They will continue to do so. In many cases these transitions are managed easily and effectively. Yet in others, they are not. There are sometimes legal and regulatory barriers to change, as well as financial incentives that act as barriers to effective working. There is a danger in sweeping all of these away, as they can provide much-needed protection for health workers, who in many countries are inadequately rewarded for their commitment and dedication. But on the other hand, there is a need for sufficient flexibility to allow them to develop as circumstances change.
The third relates to the hospital and its wider environment. It is abundantly clear that the hospital is only one part of the health system and for many patients the boundary between it and the rest of the health system can act as an impenetrable barrier. Many contemporary advances, in particular those that seek to bring sophisticated treatment to patients as quickly as possible, require models of care that reach beyond the hospital into the patient’s home. Similarly, there is a need to ensure that the process of being discharged from hospital is as smooth as possible, and is not seen simply as a means of emptying a bed for the next admission. This means that hospitals need to be planned as part of the wider system in which they sit, both in terms of the opportunities to work differently with primary care and community-based services, but also as a part of a wider network with other hospitals and specialist centres. This also means that traditional approaches that use beds as the currency for planning hospitals is now inadequate and potentially misleading or unhelpful.
The final recommendation relates to connectivity. This means connectivity within and beyond the hospital. It means connectivity through information technology but also in person. Indeed, it particularly means in person. Yet it is necessary to recognize that connectivity has a cost as well as benefits. Time spent in meetings is time not spent treating patients. Too often, meetings are organized where those attending see little point. They feel that their time is being wasted, little is relevant to them, and they spend most of the meeting on their tablets and smart phones, engaged not with those in the room but with those outside it. In time, they drift off, finding excuses to stop attending. There is a clear need to find new ways of communicating in which the benefits outweigh the costs.
It is impossible to know what the hospital of the future will look like, just as it was impossible to say what the future of travel would be before the Wright brothers took their first flight. All that can be said is that the future will be different from the present. What is important is that structures and systems are put in place that have sufficient flexibility and ability to learn as circumstances change.