Article contents
Grain size effects on Ni/Al nanolaminate combustion
Published online by Cambridge University Press: 06 March 2019
Abstract
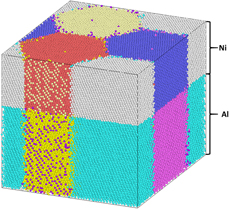
Reactions in Ni/Al nanolaminates exhibit high combustion temperatures and wave speeds that are customizable through changes to nanostructure. Nanolaminates fabricated via vapor deposition exhibit columnar grains with average diameters on the order of the individual layer thickness; yet, their role on nanolaminate combustion has not been previously investigated. The current work uses molecular dynamics simulations to elucidate the effect of grain size on reaction rates and combustion temperatures in Ni/Al nanolaminates. Decreasing grain size is shown to increase reaction rates as well as increase peak temperatures consistent with the excess enthalpy of smaller grain sizes. Additionally, grain boundaries provide heterogenous nucleation sites for the diffusion-restricting B2–NiAl phase. Focusing on Ni diffusion into liquid Al, an effective diffusion coefficient is computed as a function of grain size, which may be used in thermodynamic models for this stage of the reaction.
Keywords
- Type
- Invited Paper
- Information
- Journal of Materials Research , Volume 34 , Issue 13: Focus Issue: Intrinsic and Extrinsic Size Effects in Materials , 15 July 2019 , pp. 2229 - 2238
- Copyright
- Copyright © Materials Research Society 2019
References
- 9
- Cited by




