Article contents
Cuatrocapaite-(NH4) and cuatrocapaite-(K), two new minerals from the Torrecillas mine, Iquique Province, Chile, related to lucabindiite and gajardoite
Published online by Cambridge University Press: 22 April 2019
Abstract
The new minerals cuatrocapaite-(NH4) (IMA2018-083) and cuatrocapaite-(K) (IMA2018-084) are the NH4- and K-dominant members of a series with the general formula (NH4,K)3(NaMg□)(As2O3)6Cl6·16H2O. Both minerals were found at the Torrecillas mine, Iquique Province, Chile, where they occur as secondary alteration phases. Both minerals occur as hexagonal tablets up to ~0.3 mm in diameter. They are transparent, with a vitreous lustre and white streak. For both, the Mohs hardness is ca. 2½, the crystals are somewhat flexible, but not elastic, the fracture is irregular and the cleavage is perfect on {001}. The measured densities are 2.65(2) and 2.76(2) g/cm3 for the NH4- and K-dominant species, respectively. Optically, cuatrocapaite-(NH4) is uniaxial (–) with ω = 1.779(3) and ε = 1.541(3) and cuatrocapaite-(K) is uniaxial (–) with ω = 1.777(3) and ε = 1.539(3) (white light). The minerals are insoluble in acids, but decompose in NaOH(aq). The empirical formulas, determined from electron-microprobe analyses, are (NH4)2.48Na1.66Mg0.87K0.09(As12O18.05)Cl5.88·16.02H2O and K2.68Na1.33Mg0.93(NH4)0.31(As12O18.01)Cl6.16·16.04H2O. The minerals are trigonal, space group R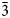 ${\bar 3}$m; the cuatrocapaite-(NH4) cell parameters are a = 5.25321(19), c = 46.6882(19) Å, V = 1115.80(9) Å3 and Z = 1; the cuatrocapaite-(K) cell parameters are a = 5.2637(15), c = 46.228(8) Å, V = 1109.2(7) Å3 and Z = 1. The structures, refined for cuatrocapaite-(NH4) to R1 = 1.78% for 544 Io > 2σI reflections, contain four types of layers: (1) a planar neutral As2O3 (arsenite) sheet; (2) an (
${\bar 3}$m; the cuatrocapaite-(NH4) cell parameters are a = 5.25321(19), c = 46.6882(19) Å, V = 1115.80(9) Å3 and Z = 1; the cuatrocapaite-(K) cell parameters are a = 5.2637(15), c = 46.228(8) Å, V = 1109.2(7) Å3 and Z = 1. The structures, refined for cuatrocapaite-(NH4) to R1 = 1.78% for 544 Io > 2σI reflections, contain four types of layers: (1) a planar neutral As2O3 (arsenite) sheet; (2) an (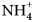 ${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $,K+) layer that links adjacent arsenite sheets; (3) a Cl– layer placed on the As side of each arsenite; and (4) a layer containing partially occupied Na, Mg and H2O sites that is flanked on either side by Cl layers. The layer sequence for the type 1, 2 and 3 layers is identical to the Cl–As2O3–K–As2O3–Cl layer sequence in the structures of lucabindiite and gajardoite.
${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $,K+) layer that links adjacent arsenite sheets; (3) a Cl– layer placed on the As side of each arsenite; and (4) a layer containing partially occupied Na, Mg and H2O sites that is flanked on either side by Cl layers. The layer sequence for the type 1, 2 and 3 layers is identical to the Cl–As2O3–K–As2O3–Cl layer sequence in the structures of lucabindiite and gajardoite.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2019
Footnotes
Associate Editor: Peter Leverett
References
- 3
- Cited by




