No CrossRef data available.
Article contents
Tennantite-(Ni), Cu6(Cu4Ni2)As4S13, from Luobusa ophiolite, Tibet, China: a new Ni member of the tetrahedrite group
Published online by Cambridge University Press: 31 May 2023
Abstract
The new mineral tennantite-(Ni), Cu6(Cu4Ni2)As4S13, has been discovered from the Luobusa Chromitite, Tibet, southwestern China. Tennantite-(Ni) occurs as anhedral grains ranging from 2 to 20 μm in size. In reflected light microscopy, tennantite-(Ni) is isotropic and appears yellow-greenish grey. Reflectance data for Commission on Ore Mineralogy wavelengths in air for tennantite-(Ni) are: 31.0 (470 nm), 29.6 (546 nm), 29.6 (589 nm) and 29.3 (650 nm). Electron microprobe analysis for holotype material gave the empirical formula (on basis of total cations = 16 apfu): M(2)Cu6 M(1)[Cu4.00(Ni0.97Cu0.53Fe0.50)Σ2.00]Σ6.00X(3)(As2.94Sb1.06)Σ4S12.77. Tennantite-(Ni) is cubic, with space group I$\bar{ 4}$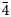 3m (#217), a =10.2957(9) Å, V = 1091.4(3) Å3 and Z = 2. By using single-crystal X-ray diffraction, the crystal structure has been determined and refined to a final R1 = 0.0423 on the basis of 163 independent reflections [Fo > 4σ (Fo)]. The calculated seven strongest powder X-ray diffraction lines [d in Å (I) (hkl)] are: 2.972 (100) (222), 1.820 (83) (440), 2.574 (28) (400), 1.552 (18) (622), 3.640 (10) (220), 1.880 (10) (521) and 1.287 (7) (800). Tennantite-(Ni) is isostructural with other tetrahedrite-group minerals, and nickel is hosted at the tetrahedrally coordinated M(1) site, along with Cu and minor Fe. The mineral and its name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2021-018).
3m (#217), a =10.2957(9) Å, V = 1091.4(3) Å3 and Z = 2. By using single-crystal X-ray diffraction, the crystal structure has been determined and refined to a final R1 = 0.0423 on the basis of 163 independent reflections [Fo > 4σ (Fo)]. The calculated seven strongest powder X-ray diffraction lines [d in Å (I) (hkl)] are: 2.972 (100) (222), 1.820 (83) (440), 2.574 (28) (400), 1.552 (18) (622), 3.640 (10) (220), 1.880 (10) (521) and 1.287 (7) (800). Tennantite-(Ni) is isostructural with other tetrahedrite-group minerals, and nickel is hosted at the tetrahedrally coordinated M(1) site, along with Cu and minor Fe. The mineral and its name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2021-018).
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2023. Published by Cambridge University Press on behalf of the Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: Koichi Momma




