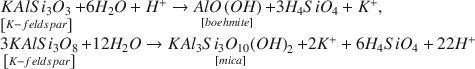The storage of CO2 in geological reservoirs requires an understanding of the impact of CO2 on clay-rich sealing cap-rocks to identify and explore critical parameters that modify petrophysical properties such as permeability and fracturing. The purpose of this study was to investigate the effect of heating, under different hydrated-CO2 partial pressures, on the chemical compositions and relative amounts of mineral phases in the Saint Martin de Bossenay (SMB, Paris Basin, France) cap-rock in order to identify possible mineral-phase transitions and to estimate reaction kinetics induced by the presence of excess dissolved CO2.
X-ray diffraction, transmission electron microscopy, and electron microprobe analyses were employed to study mineral alteration, with particular attention given to visualization and quantification of the mineral evolution of clay minerals. In all the altered mixtures investigated, the illitization of clays was combined with the formation of anhydrite. These changes were accompanied by a dolomitization and a slight increase in the quartz content. The CO2-rich samples crystallized Fe2+-and K+-enriched illites, whereas the CO2-free experiments precipitated Al3+-deprived and Mg2+-enriched illites. Advanced characterizations of cap-rock material allowed reaction paths, induced by the increase in dissolved CO2 in the porous media, to be determined precisely. The results place strong constraints on numerical models aimed at evaluating the safety of an SMB site.